Search for drugs:
Typing the drug name to query
SERTRALINE HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- During post-marketing use of sertraline, cases QTc prolongation and Torsade de Pointes (TdP) have been reported. Most reports were confounded by other risk factors. In a randomized, double-blind, placebo- and positive-controlled three-period crossover thorough QTc study in 54 healthy adult subjects, there was a positive relationship between the length of the rate-adjusted QTc interval and serum sertraline concentration. Therefore, sertraline hydrochloride should be used with caution in patients with risk factors for QTc prolongation During post-marketing use of sertraline, cases of QTc prolongation and Torsade de Pointes (TdP) have been reported. Most reports were confounded by other risk factors. In a randomized, double-blind, placebo- and positive-controlled three-period crossover thorough QTc study in 54 healthy adult subjects, there was a positive relationship between the length of the rate-adjusted QTc interval and serum sertraline concentration. Therefore, sertraline hydrochloride should be used with caution in patients with risk factors for QTc prolongation [See DRUG INTERACTIONS (7.1), CLINICAL PHARMACOLOGY (12.2)].
- DRUG INTERACTIONS
- >>91e24d17-ff0a-449c-9472-b9df74c98456.jpeg
- OVERDOSAGE
- Human Experience
- Other important adverse events reported with sertraline hydrochloride overdose (single or multiple drugs) include bradycardia, bundle branch block, coma, convulsions, delirium, hallucinations, hypertension, hypotension, manic reaction, pancreatitis, QTc-interval prolongation, Torsade de Pointes, serotonin syndrome, stupor, and syncope [See CLINICAL PHARMACOLOGY (12.2)].
- ADVERSE REACTIONS
- The following adverse reactions are described in more detail in other sections of the prescribing information:
- Hypersensitivity reactions to sertraline [See CONTRAINDICATIONS (4)]
- QTc prolongation and ventricular arrhythmias when taken with pimozide [See CONTRAINDICATIONS (4), CLINICAL PHARMACOLOGY (12.2)]
- Serotonin syndrome [See CONTRAINDICATIONS (4), WARNINGS AND PRECAUTIONS (5.2), DRUG INTERACTIONS (7.1)]
- Suicidal thoughts and behaviors [See WARNINGS AND PRECAUTIONS (5.1)]
- Increased risk of bleeding [See WARNINGS AND PRECAUTIONS (5.3)]
- Activation of mania/hypomania [See WARNINGS AND PRECAUTIONS (5.4)]
- Discontinuation syndrome [See WARNINGS AND PRECAUTIONS (5.5)]
- Seizures [See WARNINGS AND PRECAUTIONS (5.6)]
- Angle-closure glaucoma [See WARNINGS AND PRECAUTIONS (5.7)]
- Hyponatremia [See WARNINGS AND PRECAUTIONS (5.8)]
- [Post-marketing Experience]
- Cardiac disorders 鈥?AV block, bradycardia, atrial arrhythmias, QTc-interval prolongation, ventricular tachycardia (including Torsade de Pointes) [See CLINICAL PHARMACOLOGY (12.2)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of sertraline on the QTc interval was evaluated in a randomized, double-blind, placebo- and positive-controlled three-period crossover thorough QTc study in 54 healthy adult subjects. At 2-fold the maximum recommended daily dose (~3-fold the steady-state exposure for sertraline and N-desmethylsertraline), the largest mean ΔΔQTc was 10 ms with upper bound of two-sided 90% confidence interval of 12 ms. The length of the QTc interval was also positively correlated with serum concentrations of sertraline and N-desmethylsertraline concentrations. These concentration-based analyses, however, indicated a lesser effect on QTc at maximally observed concentration than in the primary analysis [See WARNINGS AND PRECAUTIONS (5), ADVERSE REACTIONS (6), DRUG INTERACTIONS (7), OVERDOSAGE (10)] .
- [Pharmacokinetics]
- Drug Interaction Studies
- Pimozide
- In a controlled study of a single dose (2 mg) of pimozide, 200 mg sertraline hydrochloride (once daily) co-administration to steady state was associated with a mean increase in pimozide AUC and C max of about 40%, but was not associated with any changes in ECG. The highest recommended pimozide dose (10 mg) has not been evaluated in combination with sertraline hydrochloride. The effect on QTc interval and PK parameters at doses higher than 2 mg of pimozide are not known [See DRUG INTERACTIONS (7.1)] .
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
247
42665
Other ADRs
49168
14068111
Odds Ratio = 1.657
Drug Property Information
ATC Code(s):
- N06AB06 - sertraline hydrochloride
- N06AB - Selective serotonin reuptake inhibitors
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:sertraline hydrochloride
Active Ingredient UNII:UTI8907Y6X
Drugbank ID:DB01104
PubChem Compound:68617
CAS Number:79617-96-2
Dosage Form(s):solution, concentrate; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 50.0 mg/day N06AB06
Chemical Structure: 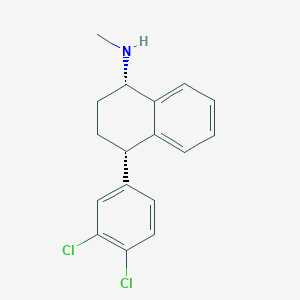

SMILE Code:
CN[C@H]1CC[C@H](C2=CC=CC=C12)C3=CC(=C(C=C3)Cl)Cl
CN[C@H]1CC[C@H](C2=CC=CC=C12)C3=CC(=C(C=C3)Cl)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.