Search for drugs:
Typing the drug name to query
ITRACONAZOLE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- Congestive Heart Failure
- TOLSURA can cause or exacerbate congestive heart failure (CHF). When itraconazole was administered intravenously to healthy human volunteers and dogs, negative inotropic effects were seen. If signs or symptoms of congestive heart failure occur or worsen during administration of TOLSURA, reassess the benefit and risk of continuing treatment [see WARNINGS AND PRECAUTIONS (5.1) and ADVERSE REACTIONS (6.1)].
- [Drug Interactions]
- Co-administration of certain drugs that are metabolized by human CYP3A4 enzymes are contraindicated with TOLSURA because plasma concentrations of such drugs are increased, which may also increase or prolong both the pharmacologic effects and/or adverse reactions to these drugs [see CONTRAINDICATIONS (4.1) and DRUG INTERACTIONS (7.1)]
- Co-administration with colchicine, fesoterodine and solifenacin is contraindicated in subjects with varying degrees of renal or hepatic impairment, and
- Co-administration with eliglustat is contraindicated in subjects that are poor or intermediate metabolizers of CYP2D6 and in subjects taking strong or moderate CYP2D6 inhibitors.
- Increased plasma concentrations of some of these drugs caused by co-administration with TOLSURA can lead to QT prolongation and/or ventricular tachyarrhythmias, including occurrences of torsades de pointes, a potentially fatal arrhythmia [see CONTRAINDICATIONS (4.1), WARNINGS AND PRECAUTIONS (5.4) and DRUG INTERACTIONS (7.1)].
- DRUG INTERACTIONS
- Effect of TOLSURA on Other Drugs
- Itraconazole and its major metabolite, hydroxy-itraconazole, are potent CYP3A4 inhibitors. Itraconazole is an inhibitor of the drug transporters P-glycoprotein and breast cancer resistance protein (BCRP). Consequently, itraconazole has the potential to interact with many concomitant drugs resulting in either increased or sometimes decreased concentrations of the concomitant drugs. Increased concentrations may increase the risk of adverse reactions associated with the concomitant drug which can be severe or life-threatening in some cases (e.g., QT prolongation, Torsade de Pointes, respiratory depression, hepatic adverse reactions, hypersensitivity reactions, myelosuppression, hypotension, seizures, angioedema, atrial fibrillation, bradycardia, priapism). Reduced concentrations of concomitant drugs may reduce their efficacy. Table 4 lists examples of drugs that may have their concentrations affected by itraconazole, but is not a comprehensive list. Refer to the approved product labeling to become familiar with the interaction pathways, risk potential, and specific actions to be taken with regards to each concomitant drug prior to initiating therapy with itraconazole.
- CONTRAINDICATIONS
- Drug Interactions
- Increased plasma concentrations of some of these drugs due to co-administration of TOLSURA can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia [see DRUG INTERACTIONS (7.1)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
81
42831
Other ADRs
6949
14110330
Odds Ratio = 3.841
Drug Property Information
ATC Code(s):
- J02AC02 - itraconazole
- J02AC - Triazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:itraconazole
Active Ingredient UNII:304NUG5GF4
Drugbank ID:DB01167
PubChem Compound:55283
CAS Number:84625-61-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 200.0 mg/day J02AC02
Chemical Structure: 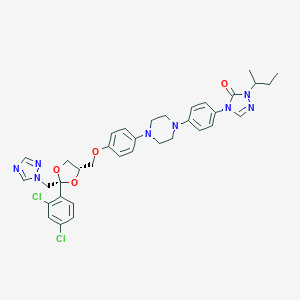

SMILE Code:
CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OC[C@H]5CO[C@](O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl
CCC(C)N1C(=O)N(C=N1)C2=CC=C(C=C2)N3CCN(CC3)C4=CC=C(C=C4)OC[C@H]5CO[C@](O5)(CN6C=NC=N6)C7=C(C=C(C=C7)Cl)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Weakness and pain in arms and legs · dark urine · history of vertebral osteomyelitis · Dx?
[Charokopos Antonios,Muhammad Tariq,Surbhi Sidana,Brateanu Andrei]J Fam Pract.2017 Mar;66(3):170-173. PMID: 28249055
2: Statin-associated rhabdomyolysis triggered by drug-drug interaction with itraconazole.
[Dybro Anne Mette,Damkier Per,Rasmussen Torsten Bloch,Hellfritzsch Maja]BMJ Case Rep.2016 Sep 7;2016. pii: bcr2016216457. doi: 10.1136/bcr-2016-216457. PMID: 27605198
3: Pharmacokinetic model for the inhibition of simvastatin metabolism by itraconazole.
[Lohitnavy Manupat,Methaneethorn Janthima,Chiang-Ngernthanyakool Rangsimaporn,Tongpeng Wasinee,Chan-Im Daranee,Phaohorm Suttipong]Conf Proc IEEE Eng Med Biol Soc.2015;2015:3246-9. doi: 10.1109/EMBC.2015.7319084. PMID: 26736984
4: Hyponatremia and antidiuresis syndrome.
[Vantyghem Marie-Christine,Balavoine Anne-Sophie,Wémeau Jean-Louis,Douillard Claire]Ann Endocrinol (Paris).2011 Dec;72(6):500-12. doi: 10.1016/j.ando.2011.10.001. Epub 2011 Nov 25. PMID: 22119069
5: Rhabdomyolysis-induced acute renal failure due to itraconazole and simvastatin association.
[Roques Sébastien,Lytrivi Maria,Rusu Daniel,Devriendt Jacques,De Bels David]Drug Metabol Drug Interact.2011;26(2):79-80. doi: 10.1515/DMDI.2011.106. Epub 2011 Apr 18. PMID: 21495875
6: [Drug interaction caused by communication problems. Rhabdomyolysis due to a combination of itraconazole and simvastatin].
[Tiessen Renger G,Lagerwey Hendrik Jan G,Jager Gea J,Sprenger Herman G]Ned Tijdschr Geneeskd.2010;154:A762. PMID: 20456775
7: Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment.
[Duncan Andrew J,Hargreaves Iain P,Damian Maxwell S,Land John M,Heales Simon J R]Toxicol Mech Methods.2009 Jan;19(1):44-50. doi: 10.1080/15376510802305047. PMID: 19778232
8: Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis.
[McKinnell James,Tayek John A]J Clin Rheumatol.2009 Sep;15(6):303-5. doi: 10.1097/RHU.0b013e3181bbbcd7. PMID: 19734738
9: Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors.
[Molden Espen,Skovlund Eva,Braathen Pia]Drug Saf.2008;31(7):587-96. PMID: 18558792
10: Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance.
[Neuvonen Pertti J,Niemi Mikko,Backman Janne T]Clin Pharmacol Ther.2006 Dec;80(6):565-81. PMID: 17178259
11: Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage.
[Wilke Russell A,Moore Jason H,Burmester James K]Pharmacogenet Genomics.2005 Jun;15(6):415-21. PMID: 15900215
12: Comparative pharmacokinetic interaction profiles of pravastatin, simvastatin, and atorvastatin when coadministered with cytochrome P450 inhibitors.
[Jacobson Terry A]Am J Cardiol.2004 Nov 1;94(9):1140-6. PMID: 15518608
13: Rhabdomyolysis after the administration of itraconazole to an asthmatic patient with bronchopulmonary aspergillosis.
[Ferrari Marcello,Bodini Ilaria,Lo Cascio Vincenzo]Respiration.2004 May-Jun;71(3):289-91. PMID: 15133351
14: Severe hypokalemia and rhabdomyolysis associated with itraconazole therapy.
[Ruiz-Contreras Jesús,Rodriguez Rocío,Gómez de Quero Pedro,González Tomé María Isabel,Sánchez Díaz Juan I]Pediatr Infect Dis J.2003 Nov;22(11):1024-5. PMID: 14628780
15: Pharmacological comparison of the statins.
[Klotz Ulrich]Arzneimittelforschung.2003;53(9):605-11. PMID: 14558433
16: Clinical pharmacokinetics of atorvastatin.
[Lennernäs Hans]Clin Pharmacokinet.2003;42(13):1141-60. PMID: 14531725
17: Effects of HMG-CoA reductase inhibitors on skeletal muscle: are all statins the same?
[Evans Marc,Rees Alan]Drug Saf.2002;25(9):649-63. PMID: 12137559
18: Itraconazole-induced rhabdomyolysis and acute renal failure in a heart transplant recipient treated with simvastatin and cyclosporine.
[Vlahakos D V,Manginas A,Chilidou D,Zamanika C,Alivizatos P A]Transplantation.2002 Jun 27;73(12):1962-4. PMID: 12131698
19: Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors.
[Williams David,Feely John]Clin Pharmacokinet.2002;41(5):343-70. PMID: 12036392
20: Rhabdomyolysis after concomitant use of cyclosporine, simvastatin, gemfibrozil, and itraconazole.
[Maxa Jan L,Melton Larry B,Ogu Chris C,Sills Michael N,Limanni Alex]Ann Pharmacother.2002 May;36(5):820-3. PMID: 11978159
21: Assessment of ADRs associated with lipid-lowering agents recorded in the Department of Internal Medicine, University Hospital, Jena.
[Hippius M,Farker K,Helble S,Hoffmann A]Int J Clin Pharmacol Ther.2002 Mar;40(3):97-101. PMID: 11911604
22: [Safety profile of statins].
[Prieto J C]Rev Med Chil.2001 Nov;129(11):1237-40. PMID: 11836874
23: The role of cytochrome P450-mediated drug-drug interactions in determining the safety of statins.
[Worz C R,Bottorff M]Expert Opin Pharmacother.2001 Jul;2(7):1119-27. PMID: 11583063
24: Drug interactions of the statins and consequences for drug selection.
[Böger R H]Int J Clin Pharmacol Ther.2001 Sep;39(9):369-82. PMID: 11563683
25: HMG-CoA reductase inhibitors and myotoxicity.
[Ucar M,Mjörndal T,Dahlqvist R]Drug Saf.2000 Jun;22(6):441-57. PMID: 10877038
26: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet.2000 Jan;38(1):41-57. PMID: 10668858
27: Rhabdomyolysis after lung transplantation.
[Malouf M A,Bicknell M,Glanville A R]Aust N Z J Med.1997 Apr;27(2):186. PMID: 9145184
28: Coadministration of itraconazole with hypolipidemic agents may induce rhabdomyolysis in healthy individuals.
[Horn M]Arch Dermatol.1996 Oct;132(10):1254. PMID: 8859048
29: Drug-interaction-induced rhabdomyolysis.
[Segaert M F,De Soete C,Vandewiele I,Verbanck J]Nephrol Dial Transplant.1996 Sep;11(9):1846-7. PMID: 8918636
30: Rhabdomyolysis from the coadministration of lovastatin and the antifungal agent itraconazole.
[Lees R S,Lees A M]N Engl J Med.1995 Sep 7;333(10):664-5. PMID: 7637734
31: High-dose itraconazole in the treatment of severe mycoses.
[Sharkey P K,Rinaldi M G,Dunn J F,Hardin T C,Fetchick R J,Graybill J R]Antimicrob Agents Chemother.1991 Apr;35(4):707-13. PMID: 1648887
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.