Search for drugs:
Typing the drug name to query
IOHEXOL
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- SEVERE ADVERSE EVENTS - INADVERTENT INTRATHECAL ADMINISTRATION
- Serious adverse reactions have been reported due to the inadvertent intrathecal administration of iodinated contrast media that are not indicated for intrathecal use. These serious adverse reactions include: death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. Special attention must be given to insure that OMNIPAQUE 140 and 350 are not administered intrathecally. (All other concentrations of OMNIPAQUE are approved for intrathecal administration).
- If grossly bloody CSF is encountered, the possible benefits of a myelographic procedure should be considered in terms of the risk to the patient.
- Caution is advised in patients with a history of epilepsy, severe cardiovascular disease, chronic alcoholism, or multiple sclerosis.
- Elderly patients may present a greater risk following myelography. The need for the procedure in these patients should be evaluated carefully. Special attention must be paid to dose and concentration of the medium, hydration, and technique used.
- Patients who are receiving anticonvulsants should be maintained on this therapy. Should a seizure occur, intravenous diazepam or phenobarbital sodium is recommended. In patients with a history of seizure activity who are not on anticonvulsant therapy, premedication with barbiturates should be considered.
- Prophylactic anticonvulsant treatment with barbiturates should be considered in patients with evidence of inadvertent intracranial entry of a large or concentrated bolus of the contrast medium since there may be an increased risk of seizure in such cases.
- Drugs which lower the seizure threshold, especially phenothiazine derivatives, including those used for their antihistamine properties, are not recommended for use with OMNIPAQUE. Others include MAO inhibitors, tri-cyclic antidepressants, CNS stimulants, and psychoactive drugs described as analeptics, major tranquilizers, or antipsychotic drugs. While the contributory role of these medications has not been established, the use of such drugs should be based on physician evaluation of potential benefits and potential risks. Physicians have discontinued these agents at least 48 hours before and for at least 24 hours postprocedure.
- Care is required in patient management to prevent inadvertent intracranial entry of a large dose or concentrated bolus of the medium. Also, effort should be directed to avoid rapid dispersion of the medium causing inadvertent rise to intracranial levels (eg, by active patient movement). Direct intracisternal or ventricular administration for standard radiography (not CT) is not recommended.
- In most reported cases of major motor seizures with nonionic myelographic media, one or more of the following factors were present. Therefore avoid:
- - Deviations from recommended procedure or in myelographic management.
- - Use in patients with a history of epilepsy.
- - Overdosage.
- - Intracranial entry of a bolus or premature diffusion of a high concentration of the medium.
- - Medication with neuroleptic drugs or phenothiazine antinauseants.
- - Failure to maintain elevation of the head during the procedure, on the stretcher, or in bed.
- - Excessive and particularly active patient movement or straining.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
10
42902
Other ADRs
4378
14112901
Odds Ratio = 0.752
Drug Property Information
ATC Code(s):
- V08AB02 - iohexol
- V08AB - "Watersoluble, nephrotropic, low osmolar X-ray contrast media"
- V08A - "X-RAY CONTRAST MEDIA, IODINATED"
- V08 - CONTRAST MEDIA
- V - VARIOUS
Active Ingredient:iohexol
Active Ingredient UNII:4419T9MX03
Drugbank ID:DB01362
PubChem Compound:3730
CAS Number:66108-95-0
Dosage Form(s):injection
Route(s) Of Administrator:intrathecal; intravascular; intravenous; oral
Daily Dose:
Chemical Structure: 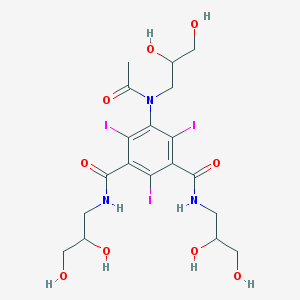

SMILE Code:
CC(=O)N(CC(CO)O)C1=C(C(=C(C(=C1I)C(=O)NCC(CO)O)I)C(=O)NCC(CO)O)I
CC(=O)N(CC(CO)O)C1=C(C(=C(C(=C1I)C(=O)NCC(CO)O)I)C(=O)NCC(CO)O)I
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Status epilepticus after myelography with iohexol (Omnipaque).
[Alimohammadi Hossein,Abdalvand Ali,Safari Saeed,Mazinanian Alireza]Am J Emerg Med.2012 Nov;30(9):2092.e1-3. doi: 10.1016/j.ajem.2011.12.034. Epub 2012 Mar 3. PMID: 22386347
2: Rhabdomyolysis after intraoperative myelography.
[Canbay Ozgür,Bal Nilüfer,Akinci Seda,Kanbak Meral,Aypar Ulkü]Paediatr Anaesth.2004 Jun;14(6):509-13. PMID: 15153217
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.