Search for drugs:
Typing the drug name to query
RISPERIDONE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Human Experience
- Premarketing experience included eight reports of acute risperidone tablets overdosage with estimated doses ranging from 20 to 300 mg and no fatalities. In general, reported signs and symptoms were those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension, and extrapyramidal symptoms. One case, involving an estimated overdose of 240 mg, was associated with hyponatremia, hypokalemia, prolonged QT, and widened QRS. Another case, involving an estimated overdose of 36 mg, was associated with a seizure.
- Postmarketing experience includes reports of acute risperidone tablets overdosage, with estimated doses of up to 360 mg. In general, the most frequently reported signs and symptoms are those resulting from an exaggeration of the drug's known pharmacological effects, i.e., drowsiness, sedation, tachycardia, hypotension, and extrapyramidal symptoms. Other adverse reactions reported since market introduction related to risperidone tablets overdose include prolonged QT interval and convulsions. Torsade de pointes has been reported in association with combined overdose of risperidone tablets and paroxetine.
- ADVERSE REACTIONS
- Clinical Trials Experience
- Changes in ECG Parameters
- Between-group comparisons for pooled placebo-controlled trials in adults revealed no statistically significant differences between risperidone and placebo in mean changes from baseline in ECG parameters, including QT, QTc, and PR intervals, and heart rate. When all risperidone tablets doses were pooled from randomized controlled trials in several indications, there was a mean increase in heart rate of 1 beat per minute compared to no change for placebo patients. In short-term schizophrenia trials, higher doses of risperidone (8-16 mg/day) were associated with a higher mean increase in heart rate compared to placebo (4-6 beats per minute). In pooled placebo-controlled acute mania trials in adults, there were small decreases in mean heart rate, similar among all treatment groups.
- In the two placebo-controlled trials in children and adolescents with autistic disorder (aged 5 – 16 years) mean changes in heart rate were an increase of 8.4 beats per minute in the risperidone tablets groups and 6.5 beats per minute in the placebo group. There were no other notable ECG changes.
- In a placebo-controlled acute mania trial in children and adolescents (aged 10 – 17 years), there were no significant changes in ECG parameters, other than the effect of risperidone tablets to transiently increase pulse rate (< 6 beats per minute). In two controlled schizophrenia trials in adolescents (aged 13 – 17 years), there were no clinically meaningful changes in ECG parameters including corrected QT intervals between treatment groups or within treatment groups over time.
- [Postmarketing Experience]
- The following adverse reactions have been identified during postapproval use of risperidone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency establish a causal relationship to drug exposure. These adverse reactions include: alopecia, anaphylactic reaction, angioedema, atrial fibrillation, cardiopulmonary arrest, diabetic ketoacidosis in patients with impaired glucose metabolism, dysgeusia, hypoglycemia, hypothermia, ileus, inappropriate antidiuretic hormone secretion, intestinal obstruction, jaundice, mania, pancreatitis, pituitary adenoma, precocious puberty, pulmonary embolism, QT prolongation, sleep apnea syndrome, somnambulism, Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), sudden death, thrombocytopenia, thrombotic thrombocytopenic purpura, urinary retention and water intoxication.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
537
42375
Other ADRs
63216
14054063
Odds Ratio = 2.818
Drug Property Information
ATC Code(s):
- N05AX08 - risperidone
- N05AX - Other antipsychotics
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:risperidone
Active Ingredient UNII:L6UH7ZF8HC
Drugbank ID:DB00734
PubChem Compound:5073
CAS Number:106266-06-2
Dosage Form(s):solution
Route(s) Of Administrator:oral
Daily Dose:
- 5.0 mg/day N05AX08
Chemical Structure: 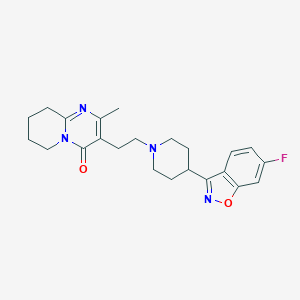

SMILE Code:
CC1=C(C(=O)N2CCCCC2=N1)CCN3CCC(CC3)C4=NOC5=C4C=CC(=C5)F
CC1=C(C(=O)N2CCCCC2=N1)CCN3CCC(CC3)C4=NOC5=C4C=CC(=C5)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: ISMP Adverse Drug Reactions: Allergic Angina Caused by Fluconazole Rhabdomyolysis Caused by Risperidone High Incidence of Hyponatremia With High-Dose Trimethoprim-Sulfamethoxazole Lithium Carbonate-Induced Hypersalivation Persistent Hemorrhage After Idarucizumab Administration.
[Mancano Michael A]Hosp Pharm.2017 Jul;52(7):455-458. doi: 10.1177/0018578717717621. Epub 2017 Sep 11. PMID: 29276272
2: A Retrospective Cohort Study of Acute Kidney Injury Risk Associated with Antipsychotics.
[Jiang Yawen,McCombs Jeffrey S,Park Susie H]CNS Drugs.2017 Apr;31(4):319-326. doi: 10.1007/s40263-017-0421-4. PMID: 28290080
3: Risperidone-Associated Rhabdomyolysis Without Neuroleptic Malignant Syndrome: A Case Report.
[Look Mei Ling,Boo Yang Liang,Chin Pek Woon,Hoo Fan Kee]J Clin Psychopharmacol.2017 Feb;37(1):105-106. doi: 10.1097/JCP.0000000000000614. PMID: 27861195
4: Paliperidone Inducing Concomitantly Syndrome of Inappropriate Antidiuretic Hormone, Neuroleptic Malignant Syndrome, and Rhabdomyolysis.
[Kaur Jaspinder,Kumar Dileep,Alfishawy Mostafa,Lopez Ricardo,Sachmechi Issac]Case Rep Crit Care.2016;2016:2587963. Epub 2016 Sep 18. PMID: 27721999
5: [Dangerous drugs: products containing synthetic chemicals].
[Kamijo Yoshito]Nihon Rinsho.2016 Feb;74(2):241-4. PMID: 26915246
6: Rhabdomyolysis With Risperidone and Escitalopram Coadministration: A Case Report.
[Mermelstein Alanna Chait,Mermelstein Joseph]J Clin Psychopharmacol.2016 Feb;36(1):97-8. doi: 10.1097/JCP.0000000000000452. PMID: 26658087
7: Rhabdomyolysis with Acute Renal Failure and Deep Vein Thrombosis Induced by Antipsychotic Drugs: A Case Report.
[Jullian-Desayes I,Roselli A,Lamy C,Alberto-Gondouin M C,Janvier N,Venturi-Maestri G]Pharmacopsychiatry.2015 Nov;48(7):265-7. doi: 10.1055/s-0035-1564088. Epub 2015 Sep 23. PMID: 26398280
8: Multidrug overdose-induced myoclonus complicated by rhabdomyolysis: possible role and mechanism of muscle toxicity of risperidone.
[Hsu Y-C,Yeh Y-W]J Clin Pharm Ther.2014 Dec;39(6):698-700. doi: 10.1111/jcpt.12205. Epub 2014 Sep 9. PMID: 25203795
9: Atypical antipsychotic drugs and the risk for acute kidney injury and other adverse outcomes in older adults: a population-based cohort study.
[Hwang Y Joseph,Dixon Stephanie N,Reiss Jeffrey P,Wald Ron,Parikh Chirag R,Gandhi Sonja,Shariff Salimah Z,Pannu Neesh,Nash Danielle M,Rehman Faisal,Garg Amit X]Ann Intern Med.2014 Aug 19;161(4):242-8. doi: 10.7326/M13-2796. PMID: 25133360
10: Serotonin syndrome after sertraline overdose in a child: a case report.
[Grenha Joana,Garrido Ana,Brito Hernani,Oliveira Maria José,Santos Fátima]Case Rep Pediatr.2013;2013:897902. doi: 10.1155/2013/897902. Epub 2013 Dec 19. PMID: 24455378
11: Risperidone: rhabdomyolysis.
Prescrire Int.2013 May;22(138):128. PMID: 23819178
12: Walking and risperidone: a rare cause of acute compartment syndrome.
[Rochcongar Goulven,Maigné Gwenola,Pineau Vincent,Hulet Christophe]Joint Bone Spine.2013 Oct;80(5):542-3. doi: 10.1016/j.jbspin.2013.02.006. Epub 2013 Apr 6. PMID: 23566661
13: Rhabdomyolysis reported for children and adolescents treated with antipsychotic medicines: a case series analysis.
[Star Kristina,Iessa Noha,Almandil Noor B,Wilton Lynda,Curran Sarah,Edwards I Ralph,Wong Ian C K]J Child Adolesc Psychopharmacol.2012 Dec;22(6):440-51. doi: 10.1089/cap.2011.0134. PMID: 23234587
14: Neuroleptic malignant syndrome versus serotonin syndrome: the search for a diagnostic tool.
[Sokoro Abdulrazaq A H,Zivot Joel,Ariano Robert E]Ann Pharmacother.2011 Sep;45(9):e50. doi: 10.1345/aph.1P787. Epub 2011 Aug 30. PMID: 21878660
15: Acute camptocormia induced by olanzapine: a case report.
[Robert Florence,Koenig Martial,Robert Aurélie,Boyer Stéphane,Cathébras Pascal,Camdessanché Jean-Philippe]J Med Case Rep.2010 Jun 25;4:192. doi: 10.1186/1752-1947-4-192. PMID: 20579377
16: A perfect storm in the emergency department.
[Yee Alan H,Wijdicks Eelco F M]Neurocrit Care.2010 Apr;12(2):258-60. doi: 10.1007/s12028-009-9309-6. PMID: 20012708
17: Hypothermia and rhabdomyolysis following olanzapine injection in an adolescent with schizophreniform disorder.
[Hung Chi-Fa,Huang Tsan-Yu,Lin Pao-Yen]Gen Hosp Psychiatry.2009 Jul-Aug;31(4):376-8. doi: 10.1016/j.genhosppsych.2008.09.009. Epub 2008 Oct 18. PMID: 19555799
18: [Rhabdomyolysis and renal failure secondary to interaction between simvastatin, ciclosporin A and risperidone in an allogeneic stem cell transplantation patient].
[Vives Susana,Batlle Montserrat,Montané Eva,Ribera Josep-María]Med Clin (Barc).2008 Nov 15;131(17):676. PMID: 19087798
19: [Rhabdomyolysis caused by the association of simvastatin and risperidone].
[Patier José Luis,Ferrere Federico,Moreno-Cobo María Angeles,Echaniz Ana]Med Clin (Barc).2007 Sep 29;129(11):439. PMID: 17927942
20: A case of pulmonary thromboembolism and rhabdomyolysis during therapy with mirtazapine and risperidone.
[Zink Mathias,Knopf Udo,Argiriou Sotiria,Kuwilsky Anna]J Clin Psychiatry.2006 May;67(5):835. PMID: 16841636
21: Rhabdomyolysis and compartment syndrome with coadministration of risperidone and simvastatin.
[Webber Michael A,Mahmud Waqar,Lightfoot Jeffery D,Shekhar Anantha]J Psychopharmacol.2004 Sep;18(3):432-4. PMID: 15358990
22: [Rhabdomyolysis associated with respiratory infection in chronic psychiatric patients during neuroleptic treatment].
[Pezza M,Busiello L,Palmese S,Cascella M,Di Domenico M G,De Robertis E]Minerva Anestesiol.2003 Jun;69(6):591-6. PMID: 14564256
23: Risperidone and severe cerivastatin-induced rhabdomyolysis.
[Giner V,Muñoz R,Redón J]J Intern Med.2002 Feb;251(2):177-8. PMID: 11905593
24: Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment.
[Meltzer H Y,Cola P A,Parsa M]Neuropsychopharmacology.1996 Oct;15(4):395-405. PMID: 8887994
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.