Search for drugs:
Typing the drug name to query
RIFAPENTINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- HIV Substudy
- Two-hundred HIV-infected patients with latent tuberculosis infection received at least one dose of study drugs in the main study and an additional 193 patients received at least one dose in the extension study (total of 393; 207 received 3RPT/INH and 186 received 9INH). Compared to the HIV-negative patients enrolled in the main study, a higher proportion of HIV-infected patients in each treatment arm experienced a treatment emergent adverse reaction, including a higher incidence of hepatotoxicity. Hepatotoxicity occurred in 3/207 (1.5%) patients in the 3RPT/INH arm and in 14/186 (7.5%) in the 9INH arm. Rifamycin hypersensitivity occurred in only one HIV-infected patient.
- Eleven deaths occurred during the 33 month follow up period (6/207 in the 3RPT/INH group and 5/186 in the 9INH group) including one death in the 9INH arm during the treatment emergent period. None of the reported deaths were considered related to treatment with study drugs or tuberculosis disease.
- Selected treatment-emergent adverse reactions reported during treatment and 60 days post-treatment in less 0.5% of the 3RPT/INH combination-therapy group in the main study are presented below by body system.
- Eye Disorders: conjunctivitis.
- Blood and Lymphatic System Disorders: leukopenia, anemia, lymphadenopathy, neutropenia.
- Gastrointestinal Disorders: nausea, diarrhea, vomiting, abdominal pain constipation, dry mouth, dyspepsia, esophageal irritation, gastritis, pancreatitis.
- General Disorders and Administration Site Conditions: fatigue, pyrexia, asthenia, chest pain, chills, feeling jittery.
- Infections and Infestations: pharyngitis, viral infection, vulvovaginal candidiasis.
- Metabolism and Nutrition Disorders: hyperglycemia, gout, hyperkalemia, decreased appetite, hyperlipidemia.
- Musculoskeletal and Connective Tissue Disorders: arthralgia, myalgia, back pain, rhabdomyolysis.
- Nervous system Disorders: dizziness, convulsion, paresthesia, headache, neuropathy peripheral, syncope.
- Psychiatric Disorders: depression, anxiety, disorientation, suicidal ideation.
- Renal and Urinary Disorders: azotemia.
- Reproductive System and Breast Disorders: vulvovaginal pruritus.
- Respiratory, Thoracic and Mediastinal Disorders: cough, dyspnea, oropharyngeal pain, asthma, bronchial hyperactivity, epistaxis.
- Skin and Subcutaneous Tissue Disorders: rash, hyperhidrosis, pruritus, urticaria.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
56
14117223
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- J04AB05 - rifapentine
- J04AB - Antibiotics
- J04A - DRUGS FOR TREATMENT OF TUBERCULOSIS
- J04 - ANTIMYCOBACTERIALS
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:rifapentine
Active Ingredient UNII:XJM390A33U
Drugbank ID:DB01201
PubChem Compound:6323497
CAS Number:61379-65-5
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 110.0 mg/day J04AB05
Chemical Structure: 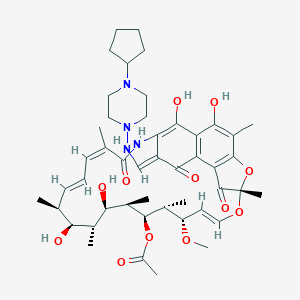

SMILE Code:
C[C@H]1/C=C/C=C(\C(=O)NC\2=C(C3=C(C(=C4C(=C3C(=O)/C2=C/NN5CCN(CC5)C6CCCC6)C(=O)[C@](O4)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)/C
C[C@H]1/C=C/C=C(\C(=O)NC\2=C(C3=C(C(=C4C(=C3C(=O)/C2=C/NN5CCN(CC5)C6CCCC6)C(=O)[C@](O4)(O/C=C/[C@@H]([C@H]([C@H]([C@@H]([C@@H]([C@@H]([C@H]1O)C)O)C)OC(=O)C)C)OC)C)C)O)O)/C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.