Search for drugs:
Typing the drug name to query
AMINOCAPROIC ACID
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Aminocaproic Acid Injection, USP contains benzyl alcohol as a preservative. The administration of medications containing benzyl alcohol as a preservative to premature neonates has been associated with a fatal “Gasping Syndrome”. (See PRECAUTIONS, PEDIATRIC USE.)
- In patients with upper urinary tract bleeding, aminocaproic acid administration has been known to cause intrarenal obstruction in the form of glomerular capillary thrombosis or clots in the renal pelvis and ureters. For this reason, aminocaproic acid should not be used in hematuria of upper urinary tract origin, unless the possible benefits outweigh the risk.
- Subendocardial hemorrhages have been observed in dogs given intravenous infusions of 0.2 times the maximum human therapeutic dose of aminocaproic acid and in monkeys given 8 times the maximum human therapeutic dose of aminocaproic acid.
- Fatty degeneration of the myocardium has been reported in dogs given intravenous doses of aminocaproic acid at 0.8 to 3.3 times the maximum human therapeutic dose and in monkeys given intravenous doses of aminocaproic acid at 6 times the maximum human therapeutic dose.
- Rarely, skeletal muscle weakness with necrosis of muscle fibers has been reported following prolonged administration. Clinical presentation may range from mild myalgias with weakness and fatigue to a severe proximal myopathy with rhabdomyolysis, myoglobinuria, and acute renal failure. Muscle enzymes, especially creatine phosphokinase (CPK) are elevated. CPK levels should be monitored in patients on long-term therapy. Aminocaproic acid administration should be stopped if a rise in CPK is noted. Resolution follows discontinuation of aminocaproic acid; however, the syndrome may recur if aminocaproic acid is restarted.
- The possibility of cardiac muscle damage should also be considered when skeletal myopathy occurs. One case of cardiac and hepatic lesions observed in man has been reported. The patient received 2 g of aminocaproic acid every 6 hours for a total dose of 26 g. Death was due to continued cerebrovascular hemorrhage. Necrotic changes in the heart and liver were noted at autopsy.
- ADVERSE REACTIONS
- Aminocaproic acid is generally well tolerated. The following adverse experiences have been reported:
- General: Edema, headache, malaise.
- Hypersensitivity Reactions: Allergic and anaphylactoid reactions, anaphylaxis.
- Local Reactions: Injection site reactions, pain and necrosis.
- Cardiovascular: Bradycardia, hypotension, peripheral ischemia, thrombosis.
- Gastrointestinal: Abdominal pain, diarrhea, nausea, vomiting.
- Hematologic: Agranulocytosis, coagulation disorder, leukopenia, thrombocytopenia.
- Musculoskeletal: CPK increased, muscle weakness, myalgia, myopathy (see WARNINGS), myositis, rhabdomyolysis.
- Neurologic: Confusion, convulsions, delirium, dizziness, hallucinations, intracranial hypertension, stroke, syncope.
- Respiratory: Dyspnea, nasal congestion, pulmonary embolism.
- Skin: Pruritus, rash.
- Special Senses: Tinnitus, vision decreased, watery eyes.
- Urogenital: BUN increased, renal failure. There have been some reports of dry ejaculation during the period of aminocaproic acid treatment. These have been reported to date only in hemophilia patients who received the drug after undergoing dental surgical procedures. However, this symptom resolved in all patients within 24 to 48 hours of completion of therapy.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
118
14117161
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- B02AA01 - aminocaproic acid
- B02AA - Amino acids
- B02A - ANTIFIBRINOLYTICS
- B02 - ANTIHEMORRHAGICS
- B - BLOOD AND BLOOD FORMING ORGANS
Active Ingredient:aminocaproic acid
Active Ingredient UNII:U6F3787206
Drugbank ID:DB00513
PubChem Compound:564
CAS Number:60-32-2
Dosage Form(s):injection, solution
Route(s) Of Administrator:intravenous
Daily Dose:
- 16000.0 mg/day B02AA01
Chemical Structure: 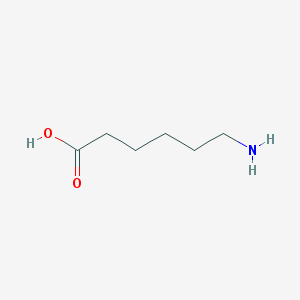

SMILE Code:
C(CCC(=O)O)CCN
C(CCC(=O)O)CCN
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis induced by epsilon-aminocaproic acid.
[Seymour B D,Rubinger M]Ann Pharmacother.1997 Jan;31(1):56-8. PMID: 8997467
2: [Drug-induced rhabdomyolysis].
[Chichmanian R M,Mignot G,Spreux A]Ann Med Interne (Paris).1991;142(8):587-91. PMID: 1807179
3: [Recurrent macroscopic hematuria and heterozygote drepanocytosis].
[Nedelec G,Didelot F,Giudicelli C P,Perrot S,Debord T,Brouard R,Rougier Y]Ann Med Interne (Paris).1987;138(1):52-5. PMID: 3592455
4: Epsilon-aminocaproic acid-induced myopathy. A case report.
[Morris C D,Jacobs P,Berman P A,Rutherfoord G S]S Afr Med J.1983 Sep 3;64(10):363-6. PMID: 6612533
5: Acute rhabdomyolysis during treatment with epsilon aminocaproic acid. Description of two cases.
[Luliri P,Bobbio-Pallavicini E,Gorini M]Haematologica.1983 Sep-Oct;68(5):664-9. PMID: 6416944
6: Myopathy induced by epsilon-aminocaproic acid. Case report.
[Brown J A,Wollmann R L,Mullan S]J Neurosurg.1982 Jul;57(1):130-4. PMID: 7086489
7: Epsilon-aminocaproic acid myopathy. Report of a case and literature review.
[Vanneste J A,van Wijngaarden G K]Eur Neurol.1982;21(4):242-8. PMID: 7117311
8: Myoglobinuria following epsilon-aminocaproic acid (EACA) therapy. Case report.
[Brodkin H M]J Neurosurg.1980 Nov;53(5):690-2. PMID: 7431078
9: [Acute rhabdomyolysis induced by aminocaproic acid].
[Le Porrier M,Foncin J F,Gazengel C,Huret J D,Billet R]Nouv Presse Med.1980 Sep 20;9(33):2347-8. PMID: 7433027
10: Rhabdomyolysis during treatment with epsilon-aminocaproic acid.
[Britt C W,Light R R,Peters B H,Schochet S S]Arch Neurol.1980 Mar;37(3):187-8. PMID: 7356431
11: Epsilon-aminocaproic acid (EACA).
[Griffin J D,Ellman L]Semin Thromb Hemost.1978 Summer;5(1):27-40. PMID: 568312
12: Myoglobinuria following aminocaproic acid administration.
[Rizza R A,Sclonick S,Conley C L]JAMA.1976 Oct 18;236(16):1845-6. PMID: 989530
13: Extensive muscle necrosis after long-term treatment with aminocaproic acid (EACA) in a case of hereditary periodic edema.
[Korsan-Bengtsen K,Ysander L,Blohmé G,Tibblin E]Acta Med Scand.1969 Apr;185(4):341-6. PMID: 4980341
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.