Search for drugs:
Typing the drug name to query
DELAVIRDINE MESYLATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Drug Interactions:
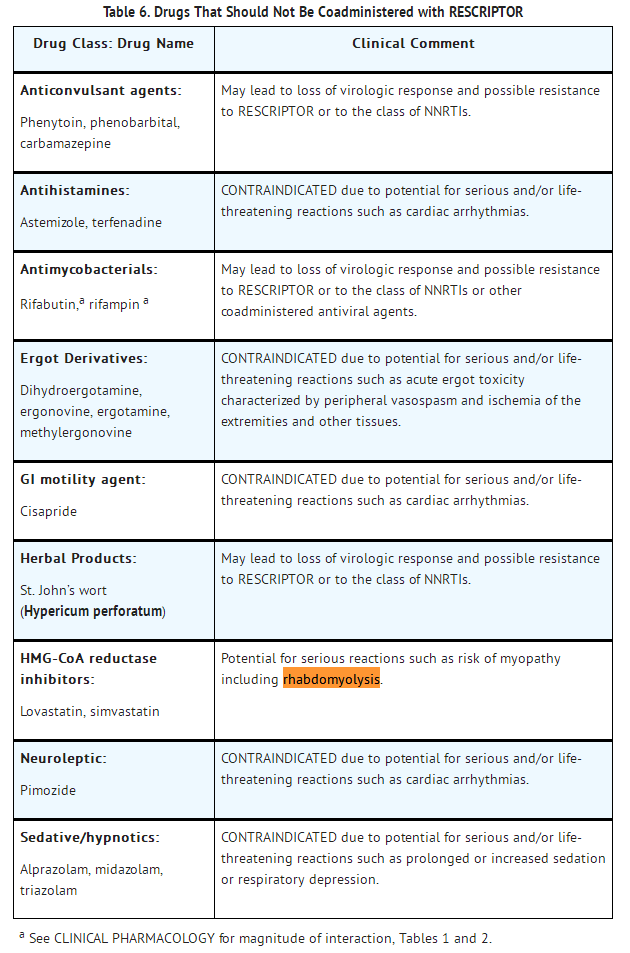
- Because delavirdine may inhibit the metabolism of many different drugs (e.g., antiarrhythmics, calcium channel blockers, sedative hypnotics, and others), serious and/or life-threatening drug interactions could result from inappropriate coadministration of some drugs with delavirdine. In addition, some drugs may markedly reduce delavirdine plasma concentrations, resulting in suboptimal antiviral activity and subsequent emergence of drug resistance. All prescribers should become familiar with the following tables in this package insert: TABLE 5, DRUGS THAT ARE CONTRAINDICATED WITH RESCRIPTOR; TABLE 6, DRUGS THAT SHOULD NOT BE COADMINISTERED WITH RESCRIPTOR; and TABLE 7, ESTABLISHED AND OTHER POTENTIALLY SIGNIFICANT DRUG INTERACTIONS: ALTERATION IN DOSE OR REGIMEN MAY BE RECOMMENDED BASED ON DRUG INTERACTION STUDIES OR PREDICTED INTERACTION. Additional details on drug interactions can be found in Tables 1 and 2 under the CLINICAL PHARMACOLOGY section.
- Concomitant use of lovastatin or simvastatin with RESCRIPTOR is not recommended. Caution should be exercised if RESCRIPTOR is used concurrently with other HMG-CoA reductase inhibitors that are also metabolized by the CYP3A4 pathway (e.g., atorvastatin or cerivastatin). The risk of myopathy including rhabdomyolysis may be increased when RESCRIPTOR is used in combination with these drugs.
- Particular caution should be used when prescribing sildenafil in patients receiving RESCRIPTOR. Coadministration of sildenafil with RESCRIPTOR is expected to substantially increase sildenafil concentrations and may result in an increase in sildenafil-associated adverse events, including hypotension, visual changes, and priapism (see PRECAUTIONS: DRUG INTERACTIONS and INFORMATION FOR PATIENTS, and the complete prescribing information for sildenafil).
- Concomitant use of St. John’s wort (Hypericum perforatum) or St. John’s wort-containing products and RESCRIPTOR is not recommended. Coadministration of St. John’s wort with NNRTIs, including RESCRIPTOR, is expected to substantially decrease NNRTI concentrations and may result in suboptimal levels of RESCRIPTOR and lead to loss of virologic response and possible resistance to RESCRIPTOR or to the class of NNRTIs.
- PRECAUTIONS
- Drug Interactions:
- (See also CONTRAINDICATIONS, WARNINGS, and CLINICAL PHARMACOLOGY: DRUG INTERACTIONS.)
- Delavirdine is an inhibitor of CYP3A isoform and other CYP isoforms to a lesser extent including CYP2C9, CYP2D6, and CYP2C19. Coadministration of RESCRIPTOR and drugs primarily metabolized by CYP3A (e.g., HMG-CoA reductase inhibitors and sildenafil) may result in increased plasma concentrations of the coadministered drug that could increase or prolong both its therapeutic or adverse effects.
- Delavirdine is metabolized primarily by CYP3A, but in vitro data suggest that delavirdine may also be metabolized by CYP2D6. Coadministration of RESCRIPTOR and drugs that induce CYP3A, such as rifampin, may decrease delavirdine plasma concentrations and reduce its therapeutic effect. Coadministration of RESCRIPTOR and drugs that inhibit CYP3A may increase delavirdine plasma concentrations.
- ADVERSE REACTIONS
- Other Adverse Events in Phase II/III Studies:
- Other adverse events that occurred in patients receiving RESCRIPTOR (in combination treatment) in all Phase II and III studies, considered possibly related to treatment, and of at least ACTG Grade 2 in intensity are listed below by body system.
- Body as a Whole: Abdominal cramps, abdominal distention, abdominal pain (localized), abscess, allergic reaction, chills, edema (generalized or localized), epidermal cyst, fever, infection, infection viral, lip edema, malaise, Mycobacterium tuberculosis infection, neck rigidity, sebaceous cyst, and redistribution/accumulation of body fat (see PRECAUTIONS: FAT REDISTRIBUTION).
- Cardiovascular System: Abnormal cardiac rate and rhythm, cardiac insufficiency, cardiomyopathy, hypertension, migraine, pallor, peripheral vascular disorder, and postural hypotension.
- Digestive System: Anorexia, bloody stool, colitis, constipation, decreased appetite, diarrhea (Clostridium difficile), diverticulitis, dry mouth, dyspepsia, dysphagia, enteritis at all levels, eructation, fecal incontinence, flatulence, gagging, gastroenteritis, gastroesophageal reflux, gastrointestinal bleeding, gastrointestinal disorder, gingivitis, gum hemorrhage, hepatomegaly, increased appetite, increased saliva, increased thirst, jaundice, mouth or tongue inflammation or ulcers, nonspecific hepatitis, oral/enteric moniliasis, pancreatitis, rectal disorder, sialadenitis, tooth abscess, and toothache.
- Hemic and Lymphatic System: Adenopathy, bruising, eosinophilia, granulocytosis, leukopenia, pancytopenia, purpura, spleen disorder, thrombocytopenia, and prolonged prothrombin time.
- Metabolic and Nutritional Disorders: Alcohol intolerance, amylase increased, bilirubinemia, hyperglycemia, hyperkalemia, hypertriglyceridemia, hyperuricemia, hypocalcemia, hyponatremia, hypophosphatemia, increased AST (SGOT), increased gamma glutamyl transpeptidase, increased lipase, increased serum alkaline phosphatase, increased serum creatinine, and weight increase or decrease.
- Musculoskeletal System: Arthralgia or arthritis of single and multiple joints, bone disorder, bone pain, myalgia, tendon disorder, tenosynovitis, tetany, and vertigo.
- Nervous System: Abnormal coordination, agitation, amnesia, change in dreams, cognitive impairment, confusion, decreased libido, disorientation, dizziness, emotional lability, euphoria, hallucination, hyperesthesia, hyperreflexia, hypertonia, hypesthesia, impaired concentration, manic symptoms, muscle cramp, nervousness, neuropathy, nystagmus, paralysis, paranoid symptoms, restlessness, sleep cycle disorder, somnolence, tingling, tremor, vertigo, and weakness.
- Respiratory System: Chest congestion, dyspnea, epistaxis, hiccups, laryngismus, pneumonia, and rhinitis.
- Skin and Appendages: Angioedema, dermal leukocytoclastic vasculitis, dermatitis, desquamation, diaphoresis, discolored skin, dry skin, erythema, erythema multiforme, folliculitis, fungal dermatitis, hair loss, herpes zoster or simplex, nail disorder, petechiae, non-application site pruritus, seborrhea, skin hypertrophy, skin disorder, skin nodule, Stevens-Johnson syndrome, urticaria, vesiculobullous rash, and wart.
- Special Senses: Blepharitis, blurred vision, conjunctivitis, diplopia, dry eyes, ear pain, parosmia, otitis media, photophobia, taste perversion, and tinnitus.
- Urogenital System: Amenorrhea, breast enlargement, calculi of the kidney, chromaturia, epididymitis, hematuria, hemospermia, impaired urination, impotence, kidney pain, metrorrhagia, nocturia, polyuria, proteinuria, testicular pain, urinary tract infection, and vaginal moniliasis.
- Postmarketing Experience:
- Adverse event terms reported from postmarketing surveillance that were not reported in the Phase II and III trials are presented below.
- Digestive System: Hepatic failure.
- Hemic and Lymphatic System: Hemolytic anemia.
- Musculoskeletal System: Rhabdomyolysis.
- Urogenital System: Acute kidney failure.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
2
42910
Other ADRs
109
14117170
Odds Ratio = 6.037
Drug Property Information
ATC Code(s):
- J05AG02 - delavirdine mesylate
- J05AG - Non-nucleoside reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:delavirdine mesylate
Active Ingredient UNII:421105KRQE
Drugbank ID:DB00705
PubChem Compound:5625
CAS Number:136817-59-9
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 1200.0 mg/day J05AG02
Chemical Structure: 

SMILE Code:
CC(C)NC1=C(N=CC=C1)N2CCN(CC2)C(=O)C3=CC4=C(N3)C=CC(=C4)NS(=O)(=O)C
CC(C)NC1=C(N=CC=C1)N2CCN(CC2)C(=O)C3=CC4=C(N3)C=CC(=C4)NS(=O)(=O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.