Search for drugs:
Typing the drug name to query
NELFINAVIR MESYLATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- >>e72c2bc6-9462-4a2e-8e1d-b97592376cbd-1.jpeg
- >>e72c2bc6-9462-4a2e-8e1d-b97592376cbd-2.jpeg
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of VIRACEPT. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Cardiovascular System: QTc prolongation, torsades de pointes.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effects on Electrocardiogram
- The effect of Viracept at the recommended dose of 1250 mg twice daily on the QTcF interval administered with a low fat meal (20% fat) was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled, crossover study in 66 healthy subjects. The maximum mean time-matched (95% upper confidence bound) differences in QTcF interval from placebo after baseline-correction was below 10 milliseconds, the threshold of clinical concern. This finding was unchanged when a single supratherapeutic dose of Viracept 3125 mg was administered following a 3-day administration of Viracept 1250 mg twice daily. The exposure at 3125 mg was 1.4-fold that at 1250 mg. The dose of 3125 mg in this study did not achieve the anticipated exposures in patients taking a high fat meal (50% fat) or with concomitant administration of drugs that could increase nelfinavir exposure [see PHARMACOKINETICS (12.3)].
- No subject in any group had an increase in QTcF of ≥60 milliseconds from baseline. No subject experienced an interval exceeding the potentially clinically relevant threshold of 500 milliseconds.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
3
42909
Other ADRs
2590
14114689
Odds Ratio = 0.382
Drug Property Information
ATC Code(s):
- J05AE04 - nelfinavir mesylate
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:nelfinavir mesylate
Active Ingredient UNII:98D603VP8V
Drugbank ID:DB00220
PubChem Compound:64143
CAS Number:159989-64-7
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 2250.0 mg/day J05AE04
Chemical Structure: 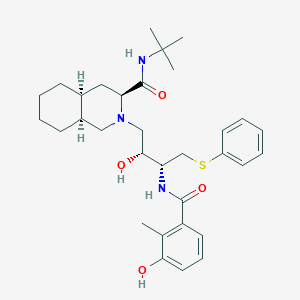

SMILE Code:
CC1=C(C=CC=C1O)C(=O)N[C@@H](CSC2=CC=CC=C2)[C@@H](CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)O
CC1=C(C=CC=C1O)C(=O)N[C@@H](CSC2=CC=CC=C2)[C@@H](CN3C[C@H]4CCCC[C@H]4C[C@H]3C(=O)NC(C)(C)C)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.