Search for drugs:
Typing the drug name to query
DANAZOL
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Drug Interactions
- Prolongation of prothrombin time occurs in patients stabilized on warfarin.
- Therapy with danazol may cause an increase in carbamazepine levels in patients taking both drugs.
- Danazol can cause insulin resistance. Caution should be exercised when used with antidiabetic drugs.
- Danazol may raise the plasma levels of cyclosporin and tacrolimus, leading to an increase of the renal toxicity of these drugs. Monitoring of systemic concentrations of these drugs and appropriate dose adjustments may be needed when used concomitantly with danazol.
- Danazol can increase the calcemic response to synthetic vitamin D analogs in primary hypoparathyroidism.
- The risk of myopathy and rhabdomyolysis is increased by concomitant administration of danazol with statins such as simvastatin, atorvastatin and lovastatin. Caution should be exercised if used concomitantly. Consult the product labeling for statin drugs for specific information on dose restrictions in presence of danazol.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
13
42899
Other ADRs
235
14117044
Odds Ratio = 18.205
Drug Property Information
ATC Code(s):
- G03XA01 - danazol
- G03XA - Antigonadotropins and similar agents
- G03X - OTHER SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM
- G03 - SEX HORMONES AND MODULATORS OF THE GENITAL SYSTEM
- G - GENITO URINARY SYSTEM AND SEX HORMONES
Active Ingredient:danazol
Active Ingredient UNII:N29QWW3BUO
Drugbank ID:DB01406
PubChem Compound:28417
CAS Number:17230-88-5
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 600.0 mg/day G03XA01
Chemical Structure: 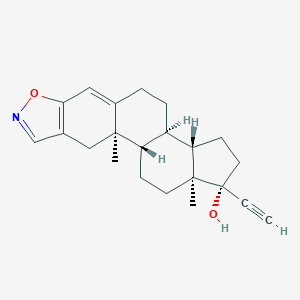

SMILE Code:
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=CC5=C(C[C@]34C)C=NO5
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@]2(C#C)O)CCC4=CC5=C(C[C@]34C)C=NO5
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhadbomyolysis associated with co-administration of danazol and lovastatin.
[Khanna Sahil,Mundell William C]Br J Clin Pharmacol.2011 Jul;72(1):166-7. doi: 10.1111/j.1365-2125.2011.03901.x. PMID: 21223355
2: Concomitant administration of simvastatin and danazol associated with fatal rhabdomyolysis.
[Stankovic Ivan,Vlahovic-Stipac Alja,Putnikovic Biljana,Cvetkovic Zorica,Neskovic Aleksandar N]Clin Ther.2010 May;32(5):909-14. doi: 10.1016/j.clinthera.2010.04.017. PMID: 20685498
3: Rhabdomyolysis and pancreatitis associated with coadministration of danazol 600 mg/d and lovastatin 40 mg/d.
[Hsieh Cheng-Yang,Chen Chih-Hung]Clin Ther.2008 Jul;30(7):1330-5. PMID: 18691993
4: Potential drug interaction between simvastatin and danazol causing rhabdomyolysis.
[Andreou E Roseann,Ledger Séadna]Can J Clin Pharmacol.2003 Winter;10(4):172-4. PMID: 14712320
5: [Severe rhabdomyolysis in a patient receiving lovastatin, danazol, and doxycycline].
[Dallaire M,Chamberland M]CMAJ.1994 Jun 15;150(12):1991-4. PMID: 8199978
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.