Search for drugs:
Typing the drug name to query
EVEROLIMUS
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- In a randomized, placebo-controlled, cross-over study, 59 healthy subjects were administered a single oral dose of Everolimus (20 mg and 50 mg) and placebo. Everolimus at single doses up to 50 mg did not prolong the QT/QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
42
42870
Other ADRs
24505
14092774
Odds Ratio = 0.564
Drug Property Information
ATC Code(s):
- L01XE10 - everolimus
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
- L04AA18 - everolimus
- L04AA - Selective immunosuppressants
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:everolimus
Active Ingredient UNII:9HW64Q8G6G
Drugbank ID:DB01590
PubChem Compound:6442177
CAS Number:159351-69-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 1.5 mg/day L04AA18
Chemical Structure: 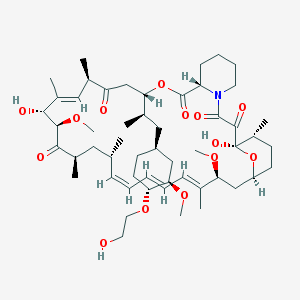

SMILE Code:
C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)OCCO)C)/C)O)OC)C)C)/C)OC
C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)OCCO)C)/C)O)OC)C)C)/C)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Ezetimibe is effective in the treatment of persistent hyperlipidemia of renal allograft recipients.
[Savvidaki E,Koukoulaki M,Benou A,Roumeliotou M,Fourtounas C,Kalliakmani P,Papachristou E,Vlachojannis J G,Goumenos D]Clin Nephrol.2011 Feb;75(2):107-12. PMID: 21255539
2: Regression of subependymal giant cell astrocytomas with RAD001 (Everolimus) in tuberous sclerosis complex.
[Yalon Michal,Ben-Sira L,Constantini S,Toren A]Childs Nerv Syst.2011 Jan;27(1):179-81. doi: 10.1007/s00381-010-1222-y. Epub 2010 Aug 12. PMID: 20703486
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.