Search for drugs:
Typing the drug name to query
ISOTRETINOIN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Information for Patients
- Patients should be informed that approximately 16% of patients treated with isotretinoin capsules in a clinical trial developed musculoskeletal symptoms (including arthralgia) during treatment. In general, these symptoms were mild to moderate, but occasionally required discontinuation of the drug. Transient pain in the chest has been reported less frequently. In the clinical trial, these symptoms generally cleared rapidly after discontinuation of isotretinoin capsules, but in some cases persisted (see ADVERSE REACTIONS: Musculoskeletal). There have been rare postmarketing reports of rhabdomyolysis, some associated with strenuous physical activity (see Laboratory Tests: CPK).
- Laboratory Tests
- CPK: Some patients undergoing vigorous physical activity while on isotretinoin capsules therapy have experienced elevated CPK levels; however, the clinical significance is unknown. There have been rare postmarketing reports of rhabdomyolysis, some associated with strenuous physical activity. In a clinical trial of 217 pediatric patients (12 to 17 years) with severe recalcitrant nodular acne, transient elevations in CPK were observed in 12% of patients, including those undergoing strenuous physical activity in association with reported musculoskeletal adverse events such as back pain, arthralgia, limb injury, or muscle sprain. In these patients, approximately half of the CPK elevations returned to normal within 2 weeks and half returned to normal within 4 weeks. No cases of rhabdomyolysis were reported in this trial.
- ADVERSE REACTIONS
- Musculoskeletal
- skeletal hyperostosis, calcification of tendons and ligaments, premature epiphyseal closure, decreases in bone mineral density (see WARNINGS: Skeletal), musculoskeletal symptoms (sometimes severe) including back pain, myalgia, and arthralgia (see PRECAUTIONS: Information for Patients), transient pain in the chest (see PRECAUTIONS: Information for Patients), arthritis, tendonitis, other types of bone abnormalities, elevations of CPK/rare reports of rhabdomyolysis (see PRECAUTIONS: Laboratory Tests).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
42
42870
Other ADRs
54940
14062339
Odds Ratio = 0.251
Drug Property Information
ATC Code(s):
- D10AD54 - isotretinoin
- D10AD - Retinoids for topical use in acne
- D10A - ANTI-ACNE PREPARATIONS FOR TOPICAL USE
- D10 - ANTI-ACNE PREPARATIONS
- D - DERMATOLOGICALS
- D10AD04 - isotretinoin
- D10AD - Retinoids for topical use in acne
- D10A - ANTI-ACNE PREPARATIONS FOR TOPICAL USE
- D10 - ANTI-ACNE PREPARATIONS
- D - DERMATOLOGICALS
- D10BA01 - isotretinoin
- D10BA - Retinoids for treatment of acne
- D10B - ANTI-ACNE PREPARATIONS FOR SYSTEMIC USE
- D10 - ANTI-ACNE PREPARATIONS
- D - DERMATOLOGICALS
Active Ingredient:isotretinoin
Active Ingredient UNII:EH28UP18IF
Drugbank ID:DB00982
PubChem Compound:5282379
CAS Number:4759-48-2
Dosage Form(s):capsule, gelatin coated; capsule, liquid filled
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day D10BA01
Chemical Structure: 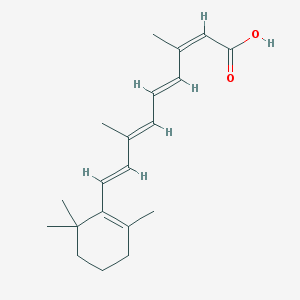

SMILE Code:
CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C\C(=O)O)/C)/C
CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C\C(=O)O)/C)/C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Rhabdomyolysis after isotretinoin treatment in a 17-year-old male].
[Paulsrud Cecilie,Stender Ida-Marie,Schmidt Lisbeth Samsø]Ugeskr Laeger.2017 Oct 2;179(40). pii: V06170462. PMID: 28992847
2: [Isotretinoin and exercise: can the two walk together?].
[Dalal Adam,Ben-Barak Shira,Zlotogorski Abraham,Constantini Naama]Harefuah.2014 Feb;153(2):104-8, 125. PMID: 24716429
3: Severe generalised rhabdomyolysis with fatal outcome associated with isotretinoin.
[Hartung Benno,Merk Hans F,Huckenbeck Wolfgang,Daldrup Thomas,Neuen-Jacob Eva,Ritz-Timme Stefanie]Int J Legal Med.2012 Nov;126(6):953-6. doi: 10.1007/s00414-012-0750-2. Epub 2012 Aug 16. PMID: 22895802
4: [Rhabdomyolysis during isotretinoin therapy].
[Gómez-Bernal S,Rodríguez-Pazos L,Rodríguez-Granados M T,Toribio J]Actas Dermosifiliogr.2011 Jun;102(5):390-1. doi: 10.1016/j.ad.2010.07.010. Epub 2011 Mar 9. PMID: 21392729
5: Neuromuscular adverse effects associated with systemic retinoid dermatotherapy: monitoring and treatment algorithm for clinicians.
[Chroni Elisabeth,Monastirli Alexandra,Tsambaos Dionysios]Drug Saf.2010 Jan 1;33(1):25-34. doi: 10.2165/11319020-000000000-00000. PMID: 20000864
6: Acute rhabdomyolysis and myoglobinuria associated with isotretinoin treatment.
[Guttman-Yassky E,Hayek T,Muchnik L,Bergman R]Int J Dermatol.2003 Jun;42(6):499-500. PMID: 12786885
7: Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin.
[Landau M,Mesterman R,Ophir J,Mevorah B,Alcalay J,Harel A,Nevo Y]Acta Derm Venereol.2001 Oct-Nov;81(5):350-2. PMID: 11800143
8: Isotretinoin induced rhabdomyolysis? A case report.
[Trauner M A,Ruben B S]Dermatol Online J.1999 Nov;5(2):2. PMID: 10673455
9: [Muscular damage during isotretinoin treatment].
[Heudes A M,Laroche L]Ann Dermatol Venereol.1998 Feb;125(2):94-7. PMID: 9747221
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.