Search for drugs:
Typing the drug name to query
GLYCOPYRROLATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- In addition, the following adverse events have been reported from post-marketing experience with glycopyrrolate: malignant hyperthermia; cardiac arrhythmias (including bradycardia, ventricular tachycardia, ventricular fibrillation); cardiac arrest; hypertension; hypotension; seizures; and respiratory arrest. Post-marketing reports have included cases of heart block and QTc interval prolongation associated with the combined use of glycopyrrolate and an anticholinesterase. Injection site reactions including pruritus, edema, erythema, and pain have also been reported.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
1
42911
Other ADRs
668
14116611
Odds Ratio = 0.493
Drug Property Information
ATC Code(s):
- R03BB06 - glycopyrrolate
- R03BB - Anticholinergics
- R03B - "OTHER DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
- R03AL04 - glycopyrrolate
- R03AL -
- R03A - "ADRENERGICS, INHALANTS"
- R03 - DRUGS FOR OBSTRUCTIVE AIRWAY DISEASES
- R - RESPIRATORY SYSTEM
- A03CA05 - glycopyrrolate
- A03CA - Synthetic anticholinergic agents in combination with psycholeptics
- A03C - ANTISPASMODICS IN COMBINATION WITH PSYCHOLEPTICS
- A03 - DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
- A03AB02 - glycopyrrolate
- A03AB - "Synthetic anticholinergics, quaternary ammonium compounds"
- A03A - DRUGS FOR FUNCTIONAL BOWEL DISORDERS
- A03 - DRUGS FOR FUNCTIONAL GASTROINTESTINAL DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:glycopyrrolate
Active Ingredient UNII:V92SO9WP2I
Drugbank ID:DB00986
PubChem Compound:9933193
CAS Number:740028-90-4
Dosage Form(s):liquid
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 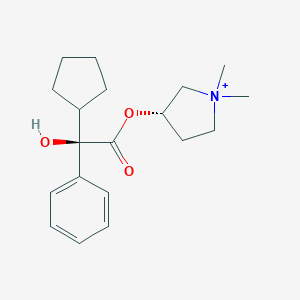

SMILE Code:
C[N+]1(CC[C@@H](C1)OC(=O)[C@@](C2CCCC2)(C3=CC=CC=C3)O)C
C[N+]1(CC[C@@H](C1)OC(=O)[C@@](C2CCCC2)(C3=CC=CC=C3)O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.