Search for drugs:
Typing the drug name to query
LOVASTATIN
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma. Strong inhibitors of CYP3A4 can raise the plasma levels of HMG-CoA reductase inhibitory activity and increase the risk of myopathy (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS).
- CONTRAINDICATIONS
- Concomitant administration with strong CYP3A4 inhibitors (e.g., itraconazole, ketoconazole, posaconazole, voriconazole, HIV protease inhibitors, boceprevir, telaprevir, erythromycin, clarithromycin, telithromycin and nefazodone) (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- WARNINGS
- Myopathy/Rhabdomyolysis
- Lovastatin, like other inhibitors of HMG-CoA reductase, occasionally causes myopathy manifested as muscle pain, tenderness or weakness with creatine kinase (CK) above ten times the upper limit of normal (ULN). Myopathy sometimes takes the form of rhabdomyolysis with or without acute renal failure secondary to myoglobinuria, and rare fatalities have occurred. The risk of myopathy is increased by high levels of HMG-CoA reductase inhibitory activity in plasma.
- As with other HMG-CoA reductase inhibitors, the risk of myopathy/rhabdomyolysis is dose related. In a clinical study (EXCEL) in which patients were carefully monitored and some interacting drugs were excluded, there was one case of myopathy among 4933 patients randomized to lovastatin 20–40 mg daily for 48 weeks, and 4 among 1649 patients randomized to 80 mg daily.
- There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
- All patients starting therapy with lovastatin, or whose dose of lovastatin is being increased, should be advised of the risk of myopathy and told to report promptly any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing lovastatin. Lovastatin therapy should be discontinued immediately if myopathy is diagnosed or suspected. In most cases, muscle symptoms and CK increases resolved when treatment was promptly discontinued. Periodic CK determinations may be considered in patients starting therapy with lovastatin or whose dose is being increased, but there is no assurance that such monitoring will prevent myopathy.
- Many of the patients who have developed rhabdomyolysis on therapy with lovastatin have had complicated medical histories, including renal insufficiency usually as a consequence of long-standing diabetes mellitus. Such patients merit closer monitoring. Lovastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. Lovastatin therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
- The risk of myopathy/rhabdomyolysis is increased by concomitant use of lovastatin with the following:
- - Strong inhibitors of CYP3A4: Lovastatin, like several other inhibitors of HMG-CoA reductase, is a substrate of cytochrome P450 3A4 (CYP3A4). Certain drugs which inhibit this metabolic pathway can raise the plasma levels of lovastatin and may increase the risk of myopathy. These include itraconazole, ketoconazole, posaconazole, voriconazole, the macrolide antibiotics erythromycin and clarithromycin, the ketolide antibiotic telithromycin, HIV protease inhibitors, boceprevir, telaprevir, or the antidepressant nefazodone. Combination of these drugs with lovastatin is contraindicated. If short-term treatment with strong CYP3A4 inhibitors is unavoidable, therapy with lovastatin should be suspended during the course of treatment (see CONTRAINDICATIONS; PRECAUTIONS, DRUG INTERACTIONS).
- - Gemfibrozil: The combined use of lovastatin with gemfibrozil should be avoided.
- - Other lipid-lowering drugs (other fibrates or ≥1 g/day of niacin): Caution should be used when prescribing other fibrates or lipid-lowering doses (≥1 g/day) of niacin with lovastatin, as these agents can cause myopathy when given alone. The benefit of further alterations in lipid levels by the combined use of lovastatin with other fibrates or niacin should be carefully weighed against the potential risks of these combinations.
- - Cyclosporine: The use of lovastatin with cyclosporine should be avoided.
- - Danazol, diltiazem, dronedarone, or verapamil with higher doses of lovastatin: The dose of lovastatin should not exceed 20 mg daily in patients receiving concomitant medication with danazol, diltiazem, dronedarone, or verapamil. The benefits of the use of lovastatin in patients receiving danazol, diltiazem, dronedarone, or verapamil should be carefully weighed against the risks of these combinations.
- - Amiodarone: The dose of lovastatin should not exceed 40 mg daily in patients receiving concomitant medication with amiodarone. The combined use of lovastatin at doses higher than 40 mg daily with amiodarone should be avoided unless the clinical benefit is likely to outweigh the increased risk of myopathy. The risk of myopathy/rhabdomyolysis is increased when amiodarone is used concomitantly with higher doses of a closely related member of the HMG-CoA reductase inhibitor class.
- - Colchicine: Cases of myopathy, including rhabdomyolysis, have been reported with lovastatin coadministered with colchicine, and caution should be exercised when prescribing lovastatin with colchicine (see PRECAUTIONS, DRUG INTERACTIONS).
- - Ranolazine: The risk of myopathy, including rhabdomyolysis, may be increased by concomitant administration of ranolazine. Dose adjustment of lovastatin may be considered during coadministration with ranolazine.
- Prescribing recommendations for interacting agents are summarized in Table VII (see also CLINICAL PHARMACOLOGY, PHARMACOKINETICS; PRECAUTIONS, DRUG INTERACTIONS; DOSAGE AND ADMINISTRATION).
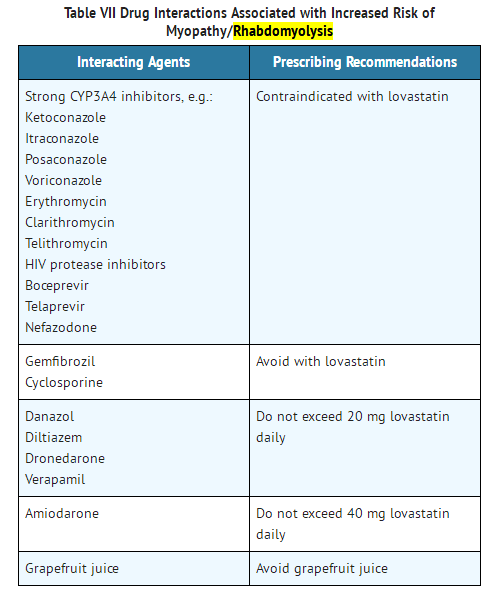
- PRECAUTIONS
- Information for Patients
- Patients should be advised about substances they should not take concomitantly with lovastatin and be advised to report promptly unexplained muscle pain, tenderness, or weakness particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing lovastatin (see list below and WARNINGS, MYOPATHY/RHABDOMYOLYSIS). Patients should also be advised to inform other physicians prescribing a new medication that they are taking lovastatin.
- Drug Interactions
- CYP3A4 Interactions
- Lovastatin is metabolized by CYP3A4 but has no CYP3A4 inhibitory activity; therefore it is not expected to affect the plasma concentrations of other drugs metabolized by CYP3A4. Strong inhibitors of CYP3A4 (e.g., itraconazole, ketoconazole, posaconazole, voriconazole, clarithromycin, telithromycin, HIV protease inhibitors, boceprevir, telaprevir, nefazodone, and erythromycin), and grapefruit juice increase the risk of myopathy by reducing the elimination of lovastatin. (See CONTRAINDICATIONS, WARNINGS, MYOPATHY/RHABDOMYOLYSIS, and CLINICAL PHARMACOLOGY, PHARMACOKINETICS.)
- Interactions With Lipid-Lowering Drugs That Can Cause Myopathy When Given Alone
- The risk of myopathy is also increased by the following lipid-lowering drugs that are not strong CYP3A4 inhibitors, but which can cause myopathy when given alone.
- See WARNINGS, MYOPATHY/RHABDOMYOLYSIS.
- Other Drug Interactions
- Cyclosporine: The risk of myopathy/rhabdomyolysis is increased by concomitant administration of cyclosporine (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Danazol, Diltiazem, Dronedarone, or Verapamil: The risk of myopathy/rhabdomyolysis is increased by concomitant administration of danazol, diltiazem, dronedarone, or verapamil particularly with higher doses of lovastatin (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS; CLINICAL PHARMACOLOGY, PHARMACOKINETICS).
- Amiodarone: The risk of myopathy/rhabdomyolysis is increased when amiodarone is used concomitantly with a closely related member of the HMG-CoA reductase inhibitor class (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Colchicine: Cases of myopathy, including rhabdomyolysis, have been reported with lovastatin coadministered with colchicine. See WARNINGS, MYOPATHY/RHABDOMYOLYSIS.
- Ranolazine: The risk of myopathy, including rhabdomyolysis, may be increased by concomitant administration of ranolazine. See WARNINGS, MYOPATHY/RHABDOMYOLYSIS.
- ADVERSE REACTIONS
- In Phase III controlled clinical studies involving 613 patients treated with lovastatin, the adverse experience profile was similar to that shown below for the 8,245-patient EXCEL study (see EXPANDED CLINICAL EVALUATION OF LOVASTATIN [EXCEL] STUDY).
- Persistent increases of serum transaminases have been noted (see WARNINGS, LIVER DYSFUNCTION). About 11% of patients had elevations of CK levels of at least twice the normal value on one or more occasions. The corresponding values for the control agent cholestyramine was 9 percent. This was attributable to the noncardiac fraction of CK. Large increases in CK have sometimes been reported (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- Concomitant Therapy
- In controlled clinical studies in which lovastatin was administered concomitantly with cholestyramine, no adverse reactions peculiar to this concomitant treatment were observed. The adverse reactions that occurred were limited to those reported previously with lovastatin or cholestyramine. Other lipid-lowering agents were not administered concomitantly with lovastatin during controlled clinical studies. Preliminary data suggests that the addition of gemfibrozil to therapy with lovastatin is not associated with greater reduction in LDL-C than that achieved with lovastatin alone. In uncontrolled clinical studies, most of the patients who have developed myopathy were receiving concomitant therapy with cyclosporine, gemfibrozil or niacin (nicotinic acid). The combined use of lovastatin with cyclosporine or gemfibrozil should be avoided. Caution should be used when prescribing other fibrates or lipid-lowering doses (≥1 g/day) of niacin with lovastatin (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with lovastatin therapy.
- Skeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias.
- There have been rare reports of immune-mediated necrotizing myopathy associated with statin use (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
- DOSAGE AND ADMINISTRATION
- Adult Patients
- The usual recommended starting dose is 20 mg once a day given with the evening meal. The recommended dosing range of lovastatin is 10–80 mg/day in single or two divided doses; the maximum recommended dose is 80 mg/day. Doses should be individualized according to the recommended goal of therapy (see NCEP GUIDELINES and CLINICAL PHARMACOLOGY). Patients requiring reductions in LDL-C of 20% or more to achieve their goal (see INDICATIONS AND USAGE) should be started on 20 mg/day of lovastatin. A starting dose of 10 mg of lovastatin may be considered for patients requiring smaller reductions. Adjustments should be made at intervals of 4 weeks or more.
- Cholesterol levels should be monitored periodically and consideration should be given to reducing the dosage of lovastatin if cholesterol levels fall significantly below the targeted range.
- Dosage in Patients taking Danazol, Diltiazem, Dronedarone, or Verapamil
- In patients taking danazol, diltiazem, dronedarone, or verapamil concomitantly with lovastatin, therapy should begin with 10 mg of lovastatin and should not exceed 20 mg/day (see CLINICAL PHARMACOLOGY, PHARMACOKINETICS, WARNINGS, MYOPATHY/RHABDOMYOLYSIS, PRECAUTIONS, DRUG INTERACTIONS, OTHER DRUG INTERACTIONS).
- Dosage in Patients taking Amiodarone
- In patients taking amiodarone concomitantly with lovastatin, the dose should not exceed 40 mg/day (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS, OTHER DRUG INTERACTIONS).
- Concomitant Lipid-Lowering Therapy
- Lovastatin is effective alone or when used concomitantly with bile-acid sequestrants (see WARNINGS, MYOPATHY/RHABDOMYOLYSIS and PRECAUTIONS, DRUG INTERACTIONS).
- Dosage in Patients with Renal Insufficiency
- In patients with severe renal insufficiency (creatinine clearance <30 mL/min), dosage increases above 20 mg/day should be carefully considered and, if deemed necessary, implemented cautiously (see CLINICAL PHARMACOLOGY and WARNINGS, MYOPATHY/RHABDOMYOLYSIS).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
226
42686
Other ADRs
1449
14115830
Odds Ratio = 51.578
Drug Property Information
ATC Code(s):
- C10BA01 - lovastatin
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10AA02 - lovastatin
- C10AA - HMG CoA reductase inhibitors
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:lovastatin
Active Ingredient UNII:9LHU78OQFD
Drugbank ID:DB00227
PubChem Compound:53232
CAS Number:75330-75-5
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 45.0 mg/day C10AA02
Chemical Structure: 

SMILE Code:
CC[C@H](C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C
CC[C@H](C)C(=O)O[C@H]1C[C@H](C=C2[C@H]1[C@H]([C@H](C=C2)C)CC[C@@H]3C[C@H](CC(=O)O3)O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Comparative impact of systemic delivery of atorvastatin, simvastatin, and lovastatin on bone mineral density of the ovariectomized rats.
[Shahrezaee Mostafa,Oryan Ahmad,Bastami Farshid,Hosseinpour Sepanta,Shahrezaee Mohammad Hossein,Kamali Amir]Endocrine.2018 Jan 25. doi: 10.1007/s12020-018-1531-6. [Epub ahead of print] PMID: 29372484
2: [Red yeast-rice-induced muscular injuries: Analysis of French pharmacovigilance database and literature review].
[Philibert Christelle,Bres Virginie,Jean-Pastor Marie-Josèphe,Guy Claire,Lebrun-Vignes Bénédicte,Robin Perrine,Pinzani Véronique,Hillaire-Buys Dominique]Therapie.2016 Oct 27. pii: S0040-5957(16)30054-3. doi: 10.2515/therapie/2015053. [Epub ahead of print] PMID: 28277227
3: Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system.
[Mazzanti Gabriela,Moro Paola Angela,Raschi Emanuel,Da Cas Roberto,Menniti-Ippolito Francesca]Br J Clin Pharmacol.2017 Apr;83(4):894-908. doi: 10.1111/bcp.13171. Epub 2017 Jan 19. PMID: 28093797
4: Statin-associated rhabdomyolysis triggered by drug-drug interaction with itraconazole.
[Dybro Anne Mette,Damkier Per,Rasmussen Torsten Bloch,Hellfritzsch Maja]BMJ Case Rep.2016 Sep 7;2016. pii: bcr2016216457. doi: 10.1136/bcr-2016-216457. PMID: 27605198
5: [Read Yeast Rice Induced Muscular Injuries: Analysis of French Pharmacovigilance Database and Literature Review].
[Philibert Christelle,Bres Virginie,Jean-Pastor Marie-Josèphe,Guy Claire,Lebrun-Vignes Bénédicte,Robin Perrine,Pinzani Véronique,Hillaire-Buys Dominique]Therapie.2015 Oct 16. [Epub ahead of print] PMID: 26475749
6: Grapefruit Juice and Statins.
[Lee Jonathan W,Morris Joan K,Wald Nicholas J]Am J Med.2016 Jan;129(1):26-9. doi: 10.1016/j.amjmed.2015.07.036. Epub 2015 Aug 20. PMID: 26299317
7: Drug-drug interactions that interfere with statin metabolism.
[Hirota Takeshi,Ieiri Ichiro]Expert Opin Drug Metab Toxicol.2015;11(9):1435-47. doi: 10.1517/17425255.2015.1056149. Epub 2015 Jun 11. PMID: 26058399
8: [Renoprotective effects of statins under the conditions of acute renal failure, caused by rhabdomyolysis].
[Zamorskiĭ I I,Zeleniuk V G]Biofizika.2014 Sep-Oct;59(5):1027-30. PMID: 25730990
9: [Renoprotective efficacy of different doses of statins in experimental acute renal failure].
[Zeleniuk V H,Zamors'kyĭ I I,Horoshko O M]Fiziol Zh.2014;60(2):75-81. PMID: 25007525
10: [Rhabdomyolysis and severe hepatotoxicity due to a drug-drug interaction between ritonavir and simvastatin. Could we use the most cost-effective statin in all human immunodeficiency virus-infected patients?].
[Bastida Carla,Also Maria Antonia,Pericas Juan Manuel,Letang Emili,Tuset Montse,Miró Josep Maria]Enferm Infecc Microbiol Clin.2014 Nov;32(9):579-82. doi: 10.1016/j.eimc.2014.03.014. Epub 2014 Jun 7. PMID: 24913991
11: Selective serotonin reuptake inhibitor drug interactions in patients receiving statins.
[Andrade Chittaranjan]J Clin Psychiatry.2014 Feb;75(2):e95-9. doi: 10.4088/JCP.13f08941. PMID: 24602259
12: Neuropsychiatric adverse events associated with statins: epidemiology, pathophysiology, prevention and management.
[Tuccori Marco,Montagnani Sabrina,Mantarro Stefania,Capogrosso-Sansone Alice,Ruggiero Elisa,Saporiti Alessandra,Antonioli Luca,Fornai Matteo,Blandizzi Corrado]CNS Drugs.2014 Mar;28(3):249-72. doi: 10.1007/s40263-013-0135-1. PMID: 24435290
13: Lipid-lowering agents for nephrotic syndrome.
[Kong Xiangyu,Yuan Hao,Fan Junming,Li Zi,Wu Taixiang,Jiang Lanhui]Cochrane Database Syst Rev.2013 Dec 10;(12):CD005425. doi: 10.1002/14651858.CD005425.pub2. PMID: 24327265
14: [Hypolipidemic agents drug interactions: approach to establish and assess its clinical significance. Structured review].
[Franco D,Henao Y,Monsalve M,Gutiérrez F,Hincapie J,Amariles P]Farm Hosp.2013 Nov-Dec;37(6):539-57. doi: 10.7399/FH.2013.37.6.1077. PMID: 24256019
15: Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study.
[Patel Amit M,Shariff Salimah,Bailey David G,Juurlink David N,Gandhi Sonja,Mamdani Muhammad,Gomes Tara,Fleet Jamie,Hwang Y Joseph,Garg Amit X]Ann Intern Med.2013 Jun 18;158(12):869-76. doi: 10.7326/0003-4819-158-12-201306180-00004. PMID: 23778904
16: Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors.
[Chauvin Benoit,Drouot Sylvain,Barrail-Tran Aurélie,Taburet Anne-Marie]Clin Pharmacokinet.2013 Oct;52(10):815-31. doi: 10.1007/s40262-013-0075-4. PMID: 23703578
17: Cytochrome P450 drug interactions with statin therapy.
[Goh Ivanna Xin Wei,How Choon How,Tavintharan Subramaniam]Singapore Med J.2013 Mar;54(3):131-5. PMID: 23546024
18: Monascus-fermented yellow pigments monascin and ankaflavin showed antiobesity effect via the suppression of differentiation and lipogenesis in obese rats fed a high-fat diet.
[Lee Chun-Lin,Wen Ja-Yan,Hsu Ya-Wen,Pan Tzu-Ming]J Agric Food Chem.2013 Feb 20;61(7):1493-500. doi: 10.1021/jf304015z. Epub 2013 Feb 8. PMID: 23360447
19: Monascin and ankaflavin have more anti-atherosclerosis effect and less side effect involving increasing creatinine phosphokinase activity than monacolin K under the same dosages.
[Lee Chun-Lin,Hung Yu-Ping,Hsu Ya-Wen,Pan Tzu-Ming]J Agric Food Chem.2013 Jan 9;61(1):143-50. doi: 10.1021/jf304346r. Epub 2012 Dec 24. PMID: 23237237
20: Red mold dioscorea: a potentially safe traditional function food for the treatment of hyperlipidemia.
[Chen Chien-Li,Pan Tzu-Ming]Food Chem.2012 Sep 15;134(2):1074-80. doi: 10.1016/j.foodchem.2012.03.019. Epub 2012 Mar 16. PMID: 23107730
21: [Psychotic Acute Episode and Rhabdomyolysis after Lovastatin Ingestion].
[Caamaño Beatriz H,Díaz Jairo M González,Bracho Daniel Guerrero,Herrera Harold,Samur Manuel Castro]Rev Colomb Psiquiatr.2012 Sep;41(3):672-9. doi: 10.1016/S0034-7450(14)60037-8. Epub 2014 May 10. PMID: 26572120
22: Acute rhabdomyolysis caused by combination therapy with atorvastatin and warfarin.
[Mackay J W,Fenech M E,Myint K S]Br J Hosp Med (Lond).2012 Feb;73(2):106-7. PMID: 22504754
23: Evidence-based prediction of statin use with lipid-panel data from the National Health and Nutrition Examination Survey.
[Gorevski Elizabeth,Bian Boyang,Kelton Christina M L,Martin Boone Jill E,Guo Jeff J]Value Health.2012 Jan;15(1):32-8. doi: 10.1016/j.jval.2011.07.005. Epub 2011 Sep 15. PMID: 22264969
24: [Rhabdomyolysis related to statin and seizures: report of 3 cases].
[Guan Yu-qing,Shi Yan-jie,Wang Qun]Nan Fang Yi Ke Da Xue Xue Bao.2011 Oct;31(10):1795-6. PMID: 22027795
25: Adverse events associated with individual statin treatments for cardiovascular disease: an indirect comparison meta-analysis.
[Alberton M,Wu P,Druyts E,Briel M,Mills E J]QJM.2012 Feb;105(2):145-57. doi: 10.1093/qjmed/hcr158. Epub 2011 Sep 14. PMID: 21920996
26: Evaluation of a pharmacist-managed amiodarone monitoring program.
[Spence Michele M,Polzin Jennifer K,Weisberger Calvin L,Martin John P,Rho Jay P,Willick Giselle H]J Manag Care Pharm.2011 Sep;17(7):513-22. PMID: 21870892
27: Rhadbomyolysis associated with co-administration of danazol and lovastatin.
[Khanna Sahil,Mundell William C]Br J Clin Pharmacol.2011 Jul;72(1):166-7. doi: 10.1111/j.1365-2125.2011.03901.x. PMID: 21223355
28: The risk for significant creatine kinase elevation with statins.
[Stolcpart Ryan S,Olson Kari L,Delate Thomas,Rasmussen Jon,Rehring Thomas F,Merenich John A]Am J Cardiovasc Drugs.2010;10(3):187-92. doi: 10.2165/11536130-000000000-00000. PMID: 20524720
29: Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins.
[Vaquero M P,Sánchez Muniz F J,Jiménez Redondo S,Prats Oliván P,Higueras F J,Bastida S]Nutr Hosp.2010 Mar-Apr;25(2):193-206. PMID: 20449528
30: Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment.
[Duncan Andrew J,Hargreaves Iain P,Damian Maxwell S,Land John M,Heales Simon J R]Toxicol Mech Methods.2009 Jan;19(1):44-50. doi: 10.1080/15376510802305047. PMID: 19778232
31: Rhabdomyolysis a result of azithromycin and statins: an unrecognized interaction.
[Strandell Johanna,Bate Andrew,Hägg Staffan,Edwards I Ralph]Br J Clin Pharmacol.2009 Sep;68(3):427-34. doi: 10.1111/j.1365-2125.2009.03473.x. PMID: 19740401
32: Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis.
[McKinnell James,Tayek John A]J Clin Rheumatol.2009 Sep;15(6):303-5. doi: 10.1097/RHU.0b013e3181bbbcd7. PMID: 19734738
33: Concurrent use of statins and amiodarone.
[Borders-Hemphill Vicky]Consult Pharm.2009 May;24(5):372-9. PMID: 19555146
34: Statin-induced muscle damage and atrogin-1 induction is the result of a geranylgeranylation defect.
[Cao Peirang,Hanai Jun-Ichi,Tanksale Preeti,Imamura Shintaro,Sukhatme Vikas P,Lecker Stewart H]FASEB J.2009 Sep;23(9):2844-54. doi: 10.1096/fj.08-128843. Epub 2009 Apr 30. PMID: 19406843
35: Rhabdomyolysis caused by a potential sitagliptin-lovastatin interaction.
[DiGregorio Robert V,Pasikhova Yanina]Pharmacotherapy.2009 Mar;29(3):352-6. doi: 10.1592/phco.29.3.352. PMID: 19249953
36: [Statin therapy and muscle disorders].
[Abel Tatjána,Fehér János]Orv Hetil.2009 Feb 8;150(6):261-3. doi: 10.1556/OH.2009.28520. PMID: 19179258
37: Should high creatine kinase discourage the initiation or continuance of statins for the treatment of hypercholesterolemia?
[Glueck Charles J,Rawal Bishal,Khan Naseer Ahmed,Yeramaneni Samrat,Goldenberg Naila,Wang Ping]Metabolism.2009 Feb;58(2):233-8. doi: 10.1016/j.metabol.2008.09.019. PMID: 19154957
38: Renal failure and rhabdomyolysis associated with sitagliptin and simvastatin use.
[Kao D P,Kohrt H E,Kugler J]Diabet Med.2008 Oct;25(10):1229-30. doi: 10.1111/j.1464-5491.2008.02536.x. PMID: 19046202
39: Myotoxicity of lipid-lowering agents in a teenager with MELAS mutation.
[Tay Stacey K H,Dimauro Salvatore,Pang Aileen Y W,Lai Poh-San,Yap Hui-Kim]Pediatr Neurol.2008 Dec;39(6):426-8. doi: 10.1016/j.pediatrneurol.2008.09.002. PMID: 19027590
40: Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients.
[Mills Edward J,Rachlis Beth,Wu Ping,Devereaux Philip J,Arora Paul,Perri Dan]J Am Coll Cardiol.2008 Nov 25;52(22):1769-81. doi: 10.1016/j.jacc.2008.08.039. PMID: 19022156
41: Rhabdomyolysis and pancreatitis associated with coadministration of danazol 600 mg/d and lovastatin 40 mg/d.
[Hsieh Cheng-Yang,Chen Chih-Hung]Clin Ther.2008 Jul;30(7):1330-5. PMID: 18691993
42: Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients.
[Martin Jill E,Cavanaugh Teresa M,Trumbull Leslie,Bass Maryetta,Weber Fredrick,Aranda-Michel Jaime,Hanaway Michael,Rudich Steven]Clin Transplant.2008 Jan-Feb;22(1):113-9. doi: 10.1111/j.1399-0012.2007.00780.x. PMID: 18217912
43: Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer: insights from large randomized statin trials.
[Alsheikh-Ali Alawi A,Maddukuri Prasad V,Han Hui,Karas Richard H]J Am Coll Cardiol.2007 Jul 31;50(5):409-18. Epub 2007 Jul 16. PMID: 17662392
44: Severe rhabdomyolysis and acute renal failure secondary to concomitant use of simvastatin, amiodarone, and atazanavir.
[Schmidt Ginelle A,Hoehns James D,Purcell Jessica L,Friedman Robert L,Elhawi Yasir]J Am Board Fam Med.2007 Jul-Aug;20(4):411-6. PMID: 17615423
45: Meta-analysis of drug-induced adverse events associated with intensive-dose statin therapy.
[Silva Matthew,Matthews Michele L,Jarvis Courtney,Nolan Nicole M,Belliveau Paul,Malloy Michael,Gandhi Pritesh]Clin Ther.2007 Feb;29(2):253-60. PMID: 17472818
46: Safety of lovastatin/extended release niacin compared with lovastatin alone, atorvastatin alone, pravastatin alone, and simvastatin alone (from the United States Food and Drug Administration adverse event reporting system).
[Alsheikh-Ali Alawi A,Karas Richard H]Am J Cardiol.2007 Feb 1;99(3):379-81. Epub 2006 Dec 8. PMID: 17261402
47: Hepatitis and rhabdomyolysis in a patient with hormone refractory prostate cancer on ketoconazole and concurrent lovastatin therapy.
[Stein C A,Goel Sanjay,Ghavamian Reza]Invest New Drugs.2007 Jun;25(3):277-8. Epub 2007 Jan 11. PMID: 17216557
48: Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance.
[Neuvonen Pertti J,Niemi Mikko,Backman Janne T]Clin Pharmacol Ther.2006 Dec;80(6):565-81. PMID: 17178259
49: Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: drug-drug interactions and interindividual differences in transporter and metabolic enzyme functions.
[Shitara Yoshihisa,Sugiyama Yuichi]Pharmacol Ther.2006 Oct;112(1):71-105. Epub 2006 May 22. PMID: 16714062
50: Statin safety: a systematic review.
[Law Malcolm,Rudnicka Alicja R]Am J Cardiol.2006 Apr 17;97(8A):52C-60C. Epub 2006 Feb 3. PMID: 16581329
51: Statin safety and drug interactions: clinical implications.
[Bottorff Michael B]Am J Cardiol.2006 Apr 17;97(8A):27C-31C. Epub 2006 Jan 25. PMID: 16581325
52: Statin-related adverse events: a meta-analysis.
[Silva Matthew A,Swanson Anna C,Gandhi Pritesh J,Tataronis Gary R]Clin Ther.2006 Jan;28(1):26-35. PMID: 16490577
53: [Fluvastatin-induced dermatomyositis].
[Thual N,Penven K,Chevallier J-M,Dompmartin A,Leroy D]Ann Dermatol Venereol.2005 Dec;132(12 Pt 1):996-9. PMID: 16446645
54: Safety of statins: effects on muscle and the liver.
[Vasudevan Abu R,Hamirani Yasmin S,Jones Peter H]Cleve Clin J Med.2005 Nov;72(11):990-3, 996-1001. PMID: 16315438
55: Frequency of myopathy in patients receiving lovastatin.
[Wortmann Robert L,Tipping Robert W,Levine Jeffrey G,Melin Jeffrey M]Am J Cardiol.2005 Apr 15;95(8):983-5. PMID: 15820170
56: Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update.
[Schachter Michael]Fundam Clin Pharmacol.2005 Feb;19(1):117-25. PMID: 15660968
57: Interactions between grapefruit juice and cardiovascular drugs.
[Bailey David G,Dresser George K]Am J Cardiovasc Drugs.2004;4(5):281-97. PMID: 15449971
58: Influence of lipid lowering fibrates on P-glycoprotein activity in vitro.
[Ehrhardt Manuela,Lindenmaier Heike,Burhenne Juergen,Haefeli Walter Emil,Weiss Johanna]Biochem Pharmacol.2004 Jan 15;67(2):285-92. PMID: 14698041
59: Pharmacological comparison of the statins.
[Klotz Ulrich]Arzneimittelforschung.2003;53(9):605-11. PMID: 14558433
60: [Statin-induced Parkinson's-syndrome. Reader's letter on the article by J. Finsterer in "Der Nervenarzt" (2003) 74:115-122].
[Müller Th]Nervenarzt.2003 Aug;74(8):726-7. Epub 2003 May 10. PMID: 12904874
61: Treating dyslipidemia with statins: the risk-benefit profile.
[Clark Luther T]Am Heart J.2003 Mar;145(3):387-96. PMID: 12660659
62: Safety and statin therapy: reconsidering the risks and benefits.
[Gotto Antonio M]Arch Intern Med.2003 Mar 24;163(6):657-9. PMID: 12639194
63: Cytochrome P450 drug interactions within the HMG-CoA reductase inhibitor class: are they clinically relevant?
[Martin Jennifer,Krum Henry]Drug Saf.2003;26(1):13-21. PMID: 12495360
64: Rhabdomyolysis due to red yeast rice (Monascus purpureus) in a renal transplant recipient.
[Prasad G V Ramesh,Wong Timothy,Meliton Galo,Bhaloo Salma]Transplantation.2002 Oct 27;74(8):1200-1. PMID: 12438974
65: Oxidation injury in patients receiving HMG-CoA reductase inhibitors: occurrence in patients without enzyme elevation or myopathy.
[Sinzinger Helmut,Chehne Fahdi,Lupattelli Graziana]Drug Saf.2002;25(12):877-83. PMID: 12241128
66: Pharmacological interactions of statins.
[Paoletti Rodolfo,Corsini Alberto,Bellosta Stefano]Atheroscler Suppl.2002 May;3(1):35-40. PMID: 12044584
67: Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors.
[Williams David,Feely John]Clin Pharmacokinet.2002;41(5):343-70. PMID: 12036392
68: Rhabdomyolysis after concomitant use of cyclosporine, simvastatin, gemfibrozil, and itraconazole.
[Maxa Jan L,Melton Larry B,Ogu Chris C,Sills Michael N,Limanni Alex]Ann Pharmacother.2002 May;36(5):820-3. PMID: 11978159
69: Assessment of ADRs associated with lipid-lowering agents recorded in the Department of Internal Medicine, University Hospital, Jena.
[Hippius M,Farker K,Helble S,Hoffmann A]Int J Clin Pharmacol Ther.2002 Mar;40(3):97-101. PMID: 11911604
70: Myopathy and rhabdomyolysis with lipid-lowering drugs.
[Hodel Christian]Toxicol Lett.2002 Mar 10;128(1-3):159-68. PMID: 11869826
71: FDA adverse event reports on statin-associated rhabdomyolysis.
[Omar Mohamed A,Wilson James P]Ann Pharmacother.2002 Feb;36(2):288-95. PMID: 11847951
72: Atorvastatin: an updated review of its pharmacological properties and use in dyslipidaemia.
[Malhotra H S,Goa K L]Drugs.2001;61(12):1835-81. PMID: 11693468
73: Metabolism and drug interactions of 3-hydroxy-3-methylglutaryl coenzyme A-reductase inhibitors (statins).
[Igel M,Sudhop T,von Bergmann K]Eur J Clin Pharmacol.2001 Aug;57(5):357-64. PMID: 11599653
74: The role of cytochrome P450-mediated drug-drug interactions in determining the safety of statins.
[Worz C R,Bottorff M]Expert Opin Pharmacother.2001 Jul;2(7):1119-27. PMID: 11583063
75: Simvastatin-diltiazem drug interaction resulting in rhabdomyolysis and hepatitis.
[Kanathur N,Mathai M G,Byrd R P,Fields C L,Roy T M]Tenn Med.2001 Sep;94(9):339-41. PMID: 11550401
76: Phase II study of high-dose lovastatin in patients with advanced gastric adenocarcinoma.
[Kim W S,Kim M M,Choi H J,Yoon S S,Lee M H,Park K,Park C H,Kang W K]Invest New Drugs.2001;19(1):81-3. PMID: 11291836
77: Rhabdomyolysis secondary to a drug interaction between simvastatin and clarithromycin.
[Lee A J,Maddix D S]Ann Pharmacother.2001 Jan;35(1):26-31. PMID: 11197581
78: Rhabdomyolysis and acute renal failure in a cardiac transplant recipient due to multiple drug interactions.
[Kusus M,Stapleton D D,Lertora J J,Simon E E,Dreisbach A W]Am J Med Sci.2000 Dec;320(6):394-7. PMID: 11149552
79: [Undesirable drug interactions of hypolipemic drugs].
[Chojnowska-Jezierska J]Pol Merkur Lekarski.2000 Sep;9(51):618-20. PMID: 11126989
80: Does differing metabolism by cytochrome p450 have clinical importance?
[Davidson M H]Curr Atheroscler Rep.2000 Jan;2(1):14-9. PMID: 11122720
81: Rhabdomyolysis in a patient taking simvastatin after addition of cyclosporine therapy.
[Hong R,Sequeira W]J Clin Rheumatol.2000 Dec;6(6):324-7. PMID: 19078494
82: HMG-CoA reductase inhibitors: assessing differences in drug interactions and safety profiles.
[Beaird S L]J Am Pharm Assoc (Wash).2000 Sep-Oct;40(5):637-44. PMID: 11029845
83: Comparative tolerability of the HMG-CoA reductase inhibitors.
[Farmer J A,Torre-Amione G]Drug Saf.2000 Sep;23(3):197-213. PMID: 11005703
84: Changes in free and esterified cholesterol: hallmarks of acute renal tubular injury and acquired cytoresistance.
[Zager R A,Kalhorn T F]Am J Pathol.2000 Sep;157(3):1007-16. PMID: 10980139
85: Development of thyroid follicular adenoma on simvastatin therapy.
[McCord E L,Goenka S]Tenn Med.2000 Jun;93(6):210-2. PMID: 10846948
86: New insights into the pharmacodynamic and pharmacokinetic properties of statins.
[Corsini A,Bellosta S,Baetta R,Fumagalli R,Paoletti R,Bernini F]Pharmacol Ther.1999 Dec;84(3):413-28. PMID: 10665838
87: 'Fire and forget?' - pharmacological considerations in coronary care.
[Bottorff M]Atherosclerosis.1999 Sep 9;147 Suppl 1:S23-30. PMID: 10575059
88: Rhabdomyolysis associated with the combined use of hydroxymethylglutaryl-coenzyme A reductase inhibitors with gemfibrozil and macrolide antibiotics.
[Landesman K A,Stozek M,Freeman N J]Conn Med.1999 Aug;63(8):455-7. PMID: 10500341
89: Case of the month: February 1999--54 year old man with severe muscle weakness.
[Hill M D,Bilbao J M]Brain Pathol.1999 Jul;9(3):607-8. PMID: 10416996
90: Atorvastatin in the treatment of primary hypercholesterolemia and mixed dyslipidemias.
[Yee H S,Fong N T]Ann Pharmacother.1998 Oct;32(10):1030-43. PMID: 9793596
91: Therapy of hyperlipidemia with lovastatin in kidney transplant patients on cyclosporine A immunosuppression: three-year experience.
[Kandus A,Kovac D,Cerne D,Koselj M,Kaplan-Pavlovcic S,Buturović J,Ponikvar R,Kveder R,Lindic J,Bren A F]Transplant Proc.1998 Jun;30(4):1307-9. PMID: 9636530
92: Drug interaction triggers weakness.
[Lilley L L,Guanci R]Am J Nurs.1998 Apr;98(4):10. PMID: 9556673
93: Multiple organ toxicity from addition of erythromycin to long-term lovastatin therapy.
[Wong P W,Dillard T A,Kroenke K]South Med J.1998 Feb;91(2):202-5. PMID: 9496876
94: [Rhabdomyolysis in a patient treated with lovastatin and cyclosporine].
[Hermida Lazcano I,Revillo Pinilla P,Nerin Sánchez C,Lechuga Durán I,Fernández López J]An Med Interna.1997 Sep;14(9):488. PMID: 9453765
95: Accumulation of lovastatin, but not pravastatin, in the blood of cyclosporine-treated kidney graft patients after multiple doses.
[Olbricht C,Wanner C,Eisenhauer T,Kliem V,Doll R,Boddaert M,O'Grady P,Krekler M,Mangold B,Christians U]Clin Pharmacol Ther.1997 Sep;62(3):311-21. PMID: 9333107
96: Safety and efficacy of long-term statin-fibrate combinations in patients with refractory familial combined hyperlipidemia.
[Athyros V G,Papageorgiou A A,Hatzikonstandinou H A,Didangelos T P,Carina M V,Kranitsas D F,Kontopoulos A G]Am J Cardiol.1997 Sep 1;80(5):608-13. PMID: 9294990
97: Rhabdomyolysis, acute renal failure and hepatopathy induced by lovastatin monotherapy.
[Chu P H,Chen W J,Chiang C W,Lee Y S]Jpn Heart J.1997 Jul;38(4):541-5. PMID: 9350151
98: HMG CoA reductase inhibitor-induced myotoxicity: pravastatin and lovastatin inhibit the geranylgeranylation of low-molecular-weight proteins in neonatal rat muscle cell culture.
[Flint O P,Masters B A,Gregg R E,Durham S K]Toxicol Appl Pharmacol.1997 Jul;145(1):99-110. PMID: 9221829
99: Lovastatin-induced rhabdomyolysis possibly associated with clarithromycin and azithromycin.
[Grunden J W,Fisher K A]Ann Pharmacother.1997 Jul-Aug;31(7-8):859-63. PMID: 9220046
100: Rhabdomyolysis associated with simvastatin-gemfibrozil therapy.
[Tal A,Rajeshawari M,Isley W]South Med J.1997 May;90(5):546-7. PMID: 9160078
101: Myositis and rhabdomyolysis associated with concurrent use of simvastatin and nefazodone.
[Jacobson R H,Wang P,Glueck C J]JAMA.1997 Jan 22-29;277(4):296-7. PMID: 9002485
102: Evaluation of fluvastatin in the treatment of hypercholesterolemia in renal transplant recipients taking cyclosporine.
[Goldberg R,Roth D]Transplantation.1996 Dec 15;62(11):1559-64. PMID: 8970607
103: Possible increased risk of rhabdomyolysis during concomitant use of simvastatin and gemfibrozil.
[van Puijenbroek E P,Du Buf-Vereijken P W,Spooren P F,van Doormaal J J]J Intern Med.1996 Dec;240(6):403-4. PMID: 9010388
104: Myoglobinuric renal failure due to long-standing lovastatin therapy in a patient with pre-existing chronic renal insufficiency.
[Biesenbach G,Janko O,Stuby U,Zazgornik J]Nephrol Dial Transplant.1996 Oct;11(10):2059-60. PMID: 8918723
105: HMG-CoA reductase inhibitor treatment in renal insufficiency.
[Wanner C]Nephrol Dial Transplant.1996 Oct;11(10):1951-2. PMID: 8918704
106: Coadministration of itraconazole with hypolipidemic agents may induce rhabdomyolysis in healthy individuals.
[Horn M]Arch Dermatol.1996 Oct;132(10):1254. PMID: 8859048
107: Drug-interaction-induced rhabdomyolysis.
[Segaert M F,De Soete C,Vandewiele I,Verbanck J]Nephrol Dial Transplant.1996 Sep;11(9):1846-7. PMID: 8918636
108: Treatment of hyperlipidemia after heart transplantation and rationale for the Heart Transplant Lipid Registry.
[Ballantyne C M,Bourge R C,Domalik L J,Eisen H J,Fishbein D P,Kubo S H,Lake K D,Radovancevic B,Taylor D O,Ventura H O,Yancy C W,Young J B]Am J Cardiol.1996 Sep 1;78(5):532-5. PMID: 8806337
109: The effect of simvastatin treatment on natural antioxidants in low-density lipoproteins and high-energy phosphates and ubiquinone in skeletal muscle.
[Laaksonen R,Jokelainen K,Laakso J,Sahi T,Harkonen M,Tikkanen M J,Himberg J J]Am J Cardiol.1996 Apr 15;77(10):851-4. PMID: 8623738
110: Effects of HMG-CoA reductase inhibitors on growth and differentiation of cultured rat skeletal muscle cells.
[Veerkamp J H,Smit J W,Benders A A,Oosterhof A]Biochim Biophys Acta.1996 Apr 12;1315(3):217-22. PMID: 8611662
111: Fluvastatin: a review of its pharmacology and use in the management of hypercholesterolaemia.
[Plosker G L,Wagstaff A J]Drugs.1996 Mar;51(3):433-59. PMID: 8882381
112: [Terminal myoglobinuric renal failure in lovastatin therapy with pre-existing chronic renal insufficiency].
[Biesenbach G,Janko O,Stuby U,Zazgornik J]Wien Klin Wochenschr.1996;108(11):334-7. PMID: 8767987
113: Pancreatitis and rhabdomyolysis associated with lovastatin-gemfibrozil therapy.
[Abdul-Ghaffar N U,el-Sonbaty M R]J Clin Gastroenterol.1995 Dec;21(4):340-1. PMID: 8583121
114: Currently available hypolipidaemic drugs and future therapeutic developments.
[Farmer J A,Gotto A M]Baillieres Clin Endocrinol Metab.1995 Oct;9(4):825-47. PMID: 8593127
115: Differential sensitivity of C2-C12 striated muscle cells to lovastatin and pravastatin.
[Gadbut A P,Caruso A P,Galper J B]J Mol Cell Cardiol.1995 Oct;27(10):2397-402. PMID: 8576954
116: Rhabdomyolysis from the coadministration of lovastatin and the antifungal agent itraconazole.
[Lees R S,Lees A M]N Engl J Med.1995 Sep 7;333(10):664-5. PMID: 7637734
117: Interactions with hydroxymethylglutaryl-coenzyme A reductase inhibitors.
[Garnett W R]Am J Health Syst Pharm.1995 Aug 1;52(15):1639-45. PMID: 7583826
118: [Rhabdomyolysis in patients treated with simvastatin and cyclosporin: role of the hepatic cytochrome P450 enzyme system activity].
[Meier C,Stey C,Brack T,Maggiorini M,Risti B,Krähenbühl S]Schweiz Med Wochenschr.1995 Jul 11;125(27-28):1342-6. PMID: 7624744
119: More on lovastatin.
[Marinella M A]West J Med.1995 Feb;162(2):176-7. PMID: 7725700
120: Dual bezafibrate-simvastatin therapy for combined hyperlipidaemia.
[Hutchesson A C,Moran A,Jones A F]J Clin Pharm Ther.1994 Dec;19(6):387-9. PMID: 7876371
121: Treatment of cerebrotendinous xanthomatosis.
[Federico A,Dotti M T]Neurology.1994 Nov;44(11):2218. PMID: 7970001
122: [The use of lovastatin in patients with hyperlipemia following heart transplantation treated with cyclosporine].
[Anguita M,Alonso-Pulpón L,Arizón J M,Vallés F]Med Clin (Barc).1994 Oct 15;103(12):477-8. PMID: 7996901
123: Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors.
[Nakahara K,Yada T,Kuriyama M,Osame M]Biochem Biophys Res Commun.1994 Aug 15;202(3):1579-85. PMID: 8060342
124: Myoglobinuric acute renal failure in a cardiac transplant patient taking lovastatin and cyclosporine.
[Alejandro D S,Petersen J]J Am Soc Nephrol.1994 Aug;5(2):153-60. PMID: 7993994
125: [Severe rhabdomyolysis in a patient receiving lovastatin, danazol, and doxycycline].
[Dallaire M,Chamberland M]CMAJ.1994 Jun 15;150(12):1991-4. PMID: 8199978
126: Rhabdomyolysis and acute renal failure in a heart transplant recipient treated with hypolipemiants.
[de Alava E,Sola J J,Lozano M D,Pardo-Mindán F J]Nephron.1994;66(2):242-3. PMID: 8139752
127: Rhabdomyolysis and acute renal failure associated with lovastatin.
[Fernández Zataraín G,Navarro V,García H,Villatoro J,Calvo C]Nephron.1994;66(4):483-4. PMID: 8015659
128: Tissue selectivity of hydroxymethylglutaryl coenzyme A (HMG CoA) reductase inhibitors.
[Sirtori C R]Pharmacol Ther.1993 Dec;60(3):431-59. PMID: 8073070
129: Rhabdomyolysis and renal failure secondary to combination therapy of hyperlipidemia with lovastatin and gemfibozil.
[Knoll R W,Ciafone R,Galen M]Conn Med.1993 Sep;57(9):593-4. PMID: 8252847
130: A prospective study on treatment of hypercholesterolemia with lovastatin in renal transplant patients receiving cyclosporine.
[Cheung A K,DeVault G A,Gregory M C]J Am Soc Nephrol.1993 Jun;3(12):1884-91. PMID: 8338920
131: [Side effects of cholesterol synthesis inhibitors].
[Wolterbeek R,Stricker B H]Ned Tijdschr Geneeskd.1993 May 8;137(19):973-7. PMID: 8497334
132: [Rhabdomyolysis caused by pravastatin and type 1 macrocreatine kinase].
[Decoulx E,Millaire A,de Groote P,Mahieux G,Ducloux G]Ann Cardiol Angeiol (Paris).1993 May;42(5):267-9. PMID: 8368799
133: Clinical pharmacokinetics and practical applications of simvastatin.
[Mauro V F]Clin Pharmacokinet.1993 Mar;24(3):195-202. PMID: 8343198
134: Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome.
[Chariot P,Abadia R,Agnus D,Danan C,Charpentier C,Gherardi R K]Am J Med.1993 Jan;94(1):109-10. PMID: 8420287
135: Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin.
[Arnadottir M,Eriksson L O,Thysell H,Karkas J D]Nephron.1993;65(3):410-3. PMID: 8289991
136: Comment: lovastatin-induced rhabdomyolysis in the absence of concomitant drugs.
[Hume A L]Ann Pharmacother.1992 Oct;26(10):1303. PMID: 1421664
137: [Acute rhabdomyolysis during treatment with simvastatin (Zocor)].
[Bertrand F,Fournier J P,Martinez P,Mahagne M H,Chichmanian R M,Ducoeur S,Lefebvre M,Avril E]Therapie.1992 Sep-Oct;47(5):442. PMID: 1299994
138: Massive rhabdomyolysis and simvastatin.
[Bizzaro N,Bagolin E,Milani L,Cereser C,Finco B]Clin Chem.1992 Aug;38(8 Pt 1):1504. PMID: 1643724
139: Worldwide experience with simvastatin/lovastatin.
[Walker J F]Eur Heart J.1992 Jul;13 Suppl B:21-2. PMID: 1644097
140: Gemfibrozil-lovastatin therapy for primary hyperlipoproteinemias.
[Glueck C J,Oakes N,Speirs J,Tracy T,Lang J]Am J Cardiol.1992 Jul 1;70(1):1-9. PMID: 1615846
141: Hyperlipidemia after heart transplantation: report of a 6-year experience, with treatment recommendations.
[Ballantyne C M,Radovancevic B,Farmer J A,Frazier O H,Chandler L,Payton-Ross C,Cocanougher B,Jones P H,Young J B,Gotto A M]J Am Coll Cardiol.1992 May;19(6):1315-21. PMID: 1564233
142: Rhabdomyolysis due to combined treatment with lovastatin and cholestyramine.
[Chrysanthopoulos C,Kounis N]BMJ.1992 May 9;304(6836):1225. PMID: 1515796
143: [Rhabdomyolysis due to simvastin. Apropos of a case with review of the literature].
[Dromer C,Vedrenne C,Billey T,Pages M,Fournié B,Fournié A]Rev Rhum Mal Osteoartic.1992 Apr;59(4):281-3. PMID: 1496277
144: Lovastatin-induced rhabdomyolysis in the absence of concomitant drugs.
[Wallace C S,Mueller B A]Ann Pharmacother.1992 Feb;26(2):190-2. PMID: 1554929
145: [Rhabdomyolysis caused by simvastatinin a patient following heart transplantation and cyclosporine therapy].
[Blaison G,Weber J C,Sachs D,Korganow A S,Martin T,Kretz J G,Pasquali J L]Rev Med Interne.1992 Jan-Feb;13(1):61-3. PMID: 1410877
146: Lovastatin in glomerulonephritis patients with hyperlipidaemia and heavy proteinuria.
[Chan P C,Robinson J D,Yeung W C,Cheng I K,Yeung H W,Tsang M T]Nephrol Dial Transplant.1992;7(2):93-9. PMID: 1314986
147: Lovastatin-associated rhabdomyolysis.
[Sylvain-Moore H,Worden J P]Heart Lung.1991 Sep;20(5 Pt 1):464-6. PMID: 1894526
148: Gemfibrozil-induced myopathy.
[Magarian G J,Lucas L M,Colley C]Arch Intern Med.1991 Sep;151(9):1873-4. PMID: 1888257
149: Expanded clinical evaluation of lovastatin (EXCEL) study results: IV. Additional perspectives on the tolerability of lovastatin.
[Dujovne C A,Chremos A N,Pool J L,Schnaper H,Bradford R H,Shear C L,Higgins J,Downton M,Franklin F A,Nash D T]Am J Med.1991 Jul 31;91(1B):25S-30S. PMID: 1831006
150: [Acute renal failure associated with miscellaneous drugs].
[Maeba T,Kondo S,Maeda A,Ishida M]Nihon Rinsho.1991 Jun;49(6):1332-6. PMID: 1909385
151: Rhabdomyolysis and simvastatin.
[Deslypere J P,Vermeulen A]Ann Intern Med.1991 Feb 15;114(4):342. PMID: 1987887
152: Rhabdomyolysis associated with lovastatin and erythromycin use.
[Spach D H,Bauwens J E,Clark C D,Burke W G]West J Med.1991 Feb;154(2):213-5. PMID: 2006579
153: Rhabdomyolysis with simvastatin use.
[Berland Y,Vacher Coponat H,Durand C,Baz M,Laugier R,Musso J L]Nephron.1991;57(3):365-6. PMID: 2017280
154: Hyperlipoproteinemia in chronic renal failure: pathophysiological and therapeutic aspects.
[Wanner C,Frommherz K,Hörl W H]Cardiology.1991;78(3):202-17. PMID: 1868498
155: Myopathy and rhabdomyolysis with lovastatin taken with gemfibrozil.
JAMA.1990 Dec 19;264(23):2991-2. PMID: 2243420
156: Rhabdomyolysis secondary to lovastatin therapy.
[Manoukian A A,Bhagavan N V,Hayashi T,Nestor T A,Rios C,Scottolini A G]Clin Chem.1990 Dec;36(12):2145-7. PMID: 2253372
157: Low-dose lovastatin safely lowers cholesterol after cardiac transplantation.
[Kobashigawa J A,Murphy F L,Stevenson L W,Moriguchi J D,Kawata N,Kamjoo P,Brownfield E,Wilmarth J,Leonard L,Chuck C]Circulation.1990 Nov;82(5 Suppl):IV281-3. PMID: 2225417
158: Myopathy and rhabdomyolysis associated with lovastatin-gemfibrozil combination therapy.
[Pierce L R,Wysowski D K,Gross T P]JAMA.1990 Jul 4;264(1):71-5. PMID: 2355431
159: Lovastatin-induced acute rhabdomyolysis.
[Kogan A D,Orenstein S]Postgrad Med J.1990 Apr;66(774):294-6. PMID: 2385552
160: Influence of lovastatin on the pharmacokinetics, toxicity and immunologic response of cyclosporine in the obese Zucker rat.
[Berens K L,Vadiei K,Brunner L J,Wasan K M,Luke D R]Biopharm Drug Dispos.1990 Apr;11(3):197-206. PMID: 2328306
161: Rhabdomyolysis and acute renal failure induced by combination lovastatin and gemfibrozil therapy.
[Marais G E,Larson K K]Ann Intern Med.1990 Feb 1;112(3):228-30. PMID: 2297197
162: Reductase inhibitor therapy of hypercholesterolemia.
[Kreisberg R A]Trans Am Clin Climatol Assoc.1991;102:153-63; discussion 163-5. PMID: 2130558
163: The role of cholesterol-lowering agents in drug-induced rhabdomyolysis and polymyositis.
[Goldman J A,Fishman A B,Lee J E,Johnson R J]Arthritis Rheum.1989 Mar;32(3):358-9. PMID: 2930608
164: [Treatment of hyperlipidemia with HMG-CoA reductase inhibitors].
[Prager R]Wien Med Wochenschr Suppl.1989;105:17-20. PMID: 2694627
165: Lovastatin and rhabdomyolysis.
[Ayanian J Z,Fuchs C S,Stone R M]Ann Intern Med.1988 Oct 15;109(8):682-3. PMID: 3421582
166: Lovastatin, nicotinic acid, and rhabdomyolysis.
[Reaven P,Witztum J L]Ann Intern Med.1988 Oct 1;109(7):597-8. PMID: 3421570
167: Rhabdomyolysis and renal injury with lovastatin use. Report of two cases in cardiac transplant recipients.
[Corpier C L,Jones P H,Suki W N,Lederer E D,Quinones M A,Schmidt S W,Young J B]JAMA.1988 Jul 8;260(2):239-41. PMID: 3290520
168: Rhabdomyolysis in patients receiving lovastatin after cardiac transplantation.
[East C,Alivizatos P A,Grundy S M,Jones P H,Farmer J A]N Engl J Med.1988 Jan 7;318(1):47-8. PMID: 3275892
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.