Search for drugs:
Typing the drug name to query
ZIDOVUDINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- In addition to adverse reactions reported from clinical trials, the following reactions have been identified during postmarketing use of zidovudine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to zidovudine.
- Body as a Whole: Back pain, chest pain, flu-like syndrome, generalized pain, redistribution/accumulation of body fat [see WARNINGS AND PRECAUTIONS (5.6)].
- Cardiovascular: Cardiomyopathy, syncope.
- Endocrine: Gynecomastia.
- Eye: Macular edema.
- Gastrointestinal: Dysphagia, flatulence, oral mucosa pigmentation, mouth ulcer.
- General: Sensitization reactions including anaphylaxis and angioedema, vasculitis.
- Hemic and Lymphatic: Aplastic anemia, hemolytic anemia, leukopenia, lymphadenopathy, pancytopenia with marrow hypoplasia, pure red cell aplasia.
- Hepatobiliary Tract and Pancreas: Hepatitis, hepatomegaly with steatosis, jaundice, lactic acidosis, pancreatitis.
- Musculoskeletal: Increased CPK, increased LDH, muscle spasm, myopathy and
- myositis with pathological changes (similar to that produced by HIV-1 disease), rhabdomyolysis, tremor.
- Nervous: Anxiety, confusion, depression, dizziness, loss of mental acuity, mania,
- paresthesia, seizures, somnolence, vertigo.
- Respiratory: Dyspnea, rhinitis, sinusitis.
- Skin: Changes in skin and nail pigmentation, pruritus, Stevens-Johnson syndrome, toxic epidermal necrolysis, sweat, urticaria.
- Special Senses: Amblyopia, hearing loss, photophobia, taste perversion.
- Urogenital: Urinary frequency, urinary hesitancy.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
16
42896
Other ADRs
4497
14112782
Odds Ratio = 1.171
Drug Property Information
ATC Code(s):
- J05AR01 - zidovudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR05 - zidovudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AF01 - zidovudine
- J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR04 - zidovudine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:zidovudine
Active Ingredient UNII:4B9XT59T7S
Drugbank ID:DB00495
PubChem Compound:35370
CAS Number:30516-87-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 600.0 mg/day J05AF01
Chemical Structure: 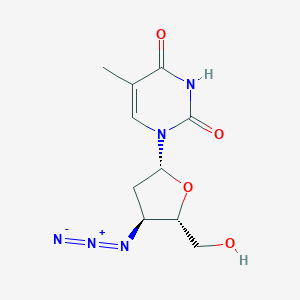

SMILE Code:
CC1=CN(C(=O)NC1=O)[C@H]2C[C@@H]([C@H](O2)CO)N=[N+]=[N-]
CC1=CN(C(=O)NC1=O)[C@H]2C[C@@H]([C@H](O2)CO)N=[N+]=[N-]
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Skeletal muscle toxicity in HIV-1-infected patients treated with a raltegravir-containing antiretroviral therapy: a cohort study.
[Calza Leonardo,Danese Ilaria,Colangeli Vincenzo,Vandi Giacomo,Manfredi Roberto,Girometti Nicolò,Borderi Marco,Appolloni Lucia,Puggioli Cristina,Viale Pierluigi]AIDS Res Hum Retroviruses.2014 Dec;30(12):1162-9. doi: 10.1089/aid.2014.0113. PMID: 25369244
2: Tolerability of HIV postexposure prophylaxis with tenofovir/emtricitabine and lopinavir/ritonavir tablet formulation.
[Tosini William,Muller Philippe,Prazuck Thierry,Benabdelmoumen Ghania,Peyrouse Eric,Christian Bernard,Quertainmont Yann,Bouvet Elisabeth,Rabaud Christian]AIDS.2010 Sep 24;24(15):2375-80. doi: 10.1097/QAD.0b013e32833dfad1. PMID: 20729709
3: Rhabdomyolysis: an evaluation of 475 hospitalized patients.
[Melli Giorgia,Chaudhry Vinay,Cornblath David R]Medicine (Baltimore).2005 Nov;84(6):377-85. PMID: 16267412
4: Skeletal muscle involvement in human immunodeficiency virus (HIV)-infected patients in the era of highly active antiretroviral therapy (HAART).
[Authier François-Jérôme,Chariot Patrick,Gherardi Romain K]Muscle Nerve.2005 Sep;32(3):247-60. PMID: 15902690
5: Anti-retrovirals and immunosuppressive drug interactions in a HIV-positive patient after liver transplantation.
[Antonini Mario,Ettorre Giuseppe Maria,Vennarecci Giovanni,D'Offizi Gianpiero,Narciso Pasquale,Del Nonno Franca,Perracchio Letizia,Visco Giuseppe,Santoro Eugenio]Hepatogastroenterology.2004 May-Jun;51(57):646-8. PMID: 15143883
6: [Severe lactic acidosis in HIV-infected patients treated with nucleosidic reverse transcriptase analogs: a report of 9 cases].
[Bonnet F,Bonarek M,Abridj A,Mercié P,Dupon M,Gemain M-C,Malvy D,Bernard N,Pellegrin J-L,Morlat P,Beylot J]Rev Med Interne.2003 Jan;24(1):11-6. PMID: 12614853
7: AIDS-related myopathy.
[Sheikh Rafiq A.,Yasmeen Shagufta,Munn Robert,Ruebner Boris H.,Ellis William G.]Med Electron Microsc.1999 Sep;32(2):79-86. PMID: 11810429
8: From the Centers for Disease Control and Prevention. Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
JAMA.2001 Jan 24-31;285(4):402-3. PMID: 11263401
9: Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
[Centers for Disease Control and Prevention (CDC)]MMWR Morb Mortal Wkly Rep.2001 Jan 5;49(51-52):1153-6. PMID: 11198946
10: Influenza myositis and rhabdomyolysis in an HIV positive man: was zidovudine a co-factor?
[Guha I,Brook M G]Sex Transm Infect.1999 Jun;75(3):204-5. PMID: 10448412
11: [Muscular complications in HIV infection].
[Authier F J,Chariot P,Gherardi R]Arch Anat Cytol Pathol.1997;45(2-3):174-8. PMID: 9382610
12: [Muscular involvement in HIV infection].
[Gherardi R,Chariot P,Authier F J]Rev Neurol (Paris).1995 Nov;151(11):603-7. PMID: 8745623
13: Rhabdomyolysis and Staphylococcus aureus septicemia in a man with the acquired immunodeficiency syndrome.
[Wu A W,Benirschke K,McCutchan J A]West J Med.1990 Jun;152(6):716-9. PMID: 2353480
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.