Search for drugs:
Typing the drug name to query
TRIENTINE HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Clinical experience with Syprine has been limited. The following adverse reactions have been reported in a clinical study in patients with Wilson's disease who were on therapy with trientine hydrochloride: iron deficiency, systemic lupus erythematosus (see CLINICAL PHARMACOLOGY). In addition, the following adverse reactions have been reported in marketed use: dystonia, muscular spasm, myasthenia gravis.
- Syprine is not indicated for treatment of biliary cirrhosis, but in one study of 4 patients treated with trientine hydrochloride for primary biliary cirrhosis, the following adverse reactions were reported: heartburn; epigastric pain and tenderness; thickening, fissuring and flaking of the skin; hypochromic microcytic anemia; acute gastritis; aphthoid ulcers; abdominal pain; melena; anorexia; malaise; cramps; muscle pain; weakness; rhabdomyolysis. A causal relationship of these reactions to drug therapy could not be rejected or established.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
50
14117229
Odds Ratio = N/A
Drug Property Information
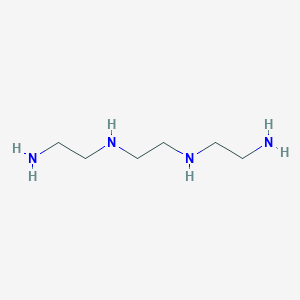
SMILE Code:
C(CNCCNCCN)N
C(CNCCNCCN)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.