Search for drugs:
Typing the drug name to query
SAQUINAVIR MESYLATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- PR Interval Prolongation
- INVIRASE/ritonavir prolongs the PR interval in a dose-dependent fashion. Cases of second or third degree atrioventricular block have been reported rarely. Patients with underlying structural heart disease, pre-existing conduction system abnormalities, cardiomyopathies and ischemic heart disease may be at increased risk for developing cardiac conduction abnormalities. ECG monitoring is recommended in these patients [see WARNINGS AND PRECAUTIONS (5.4)]. Discontinue INVIRASE/ritonavir if significant arrhythmias, QT or PR prolongation occur.
- The impact on the PR interval of coadministration of INVIRASE/ritonavir with other drugs that prolong the PR interval (including calcium channel blockers, beta-adrenergic blockers, digoxin and atazanavir) has not been evaluated. As a result, coadministration of INVIRASE/ritonavir with these drugs should be undertaken with caution, particularly with those drugs metabolized by CYP3A, and clinical monitoring is recommended [see CLINICAL PHARMACOLOGY (12.2)].
- For concomitantly used drugs, including antiretroviral agents used in combination with INVIRASE/ritonavir, physicians should refer to the complete product information for these drugs for dose adjustment recommendations and for information regarding drug-associated adverse reactions.
- [QT Interval Prolongation]
- INVIRASE/ritonavir causes dose-dependent QT prolongation. Torsade de pointes has been reported rarely post-marketing. Avoid INVIRASE/ritonavir in patients with long QT syndrome. ECG monitoring is recommended if therapy is initiated in patients with congestive heart failure, bradyarrhythmias, hepatic impairment and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating INVIRASE/ritonavir and monitor these electrolytes periodically during therapy. Do not use in combination with drugs that both increase saquinavir plasma concentrations and prolong the QT interval (see TABLE 2) [see CLINICAL PHARMACOLOGY (12.2)]. For concomitantly used drugs, including antiretroviral agents used in combination with INVIRASE/ritonavir, refer to the complete product information for these drugs for dose adjustment recommendations and for information regarding drug-associated adverse reactions [see CONTRAINDICATIONS (4) and DRUG INTERACTIONS (7)]. Discontinue INVIRASE/ritonavir if significant arrhythmias, QT or PR prolongation occurs.
- Patients initiating therapy with INVIRASE/ritonavir:
- An ECG should be performed prior to initiation of treatment. Patients with a QT interval ≥ 450 msec should not initiate treatment with INVIRASE/ritonavir.
- Treatment-naïve patients initiating treatment with INVIRASE/ritonavir should receive a reduced starting dose of INVIRASE 500 mg twice daily with ritonavir 100 mg twice daily for the first 7 days of treatment followed by INVIRASE/ritonavir 1000/100 mg twice daily due to potential for an increased risk of PR and QT interval prolongation with the standard 1000/100 mg twice daily dose [see CLINICAL PHARMACOLOGY (12.2)].
- For patients with a baseline QT interval < 450 msec, an on-treatment ECG is recommended after approximately 10 days of therapy.
- Discontinue INVIRASE/ritonavir in patients with a QT interval prolongation > 20 msec over pre-treatment.
- Patients requiring treatment with medications with the potential to increase the QT interval and concomitant INVIRASE/ritonavir:
- Such combinations should only be used where no alternative therapy is available, and the potential benefits outweigh the potential risks. An ECG should be performed prior to initiation of the concomitant therapy, and patients with a QT interval > 450 msec should not initiate the concomitant therapy. If baseline QT interval < 450 msec, an on-treatment ECG should be performed after 3–4 days of therapy. For patients demonstrating a subsequent increase in QT interval by > 20 msec after commencing concomitant therapy, the physician should use best clinical judgment to discontinue either INVIRASE/ritonavir or the concomitant therapy or both.
- A cardiology consult is recommended if drug discontinuation or interruption is being considered on the basis of ECG assessment.
- DRUG INTERACTIONS
- Established and Other Potentially Significant Drug Interactions
- Based on the finding of dose-dependent prolongations of QT and PR intervals in healthy volunteers receiving INVIRASE/ritonavir, additive effects on QT and/or PR interval prolongation may occur with certain members of the following drug classes: antiarrhythmics class IA or class III, neuroleptics, antidepressants, PDE5 inhibitors (when used for pulmonary arterial hypertension), antimicrobials, antihistaminics and others. This effect might lead to an increased risk of ventricular arrhythmias, notably torsade de pointes. Therefore, concurrent administration of these agents with INVIRASE/ritonavir is contraindicated [see CONTRAINDICATIONS (4)].
- >>c00d1607-ac36-457b-a34b-75ad74f9cf0a-1.jpeg
- >>c00d1607-ac36-457b-a34b-75ad74f9cf0a-2.jpeg
- CONTRAINDICATIONS
- INVIRASE/ritonavir is contraindicated in patients with congenital long QT syndrome, those with refractory hypokalemia or hypomagnesemia, and in combination with drugs that both increase saquinavir plasma concentrations and prolong the QT interval [see WARNINGS AND PRECAUTIONS (5.4) and CLINICAL PHARMACOLOGY (12.2)].
- DOSAGE AND ADMINISTRATION
- Recommended Dose
- Pediatric dose recommendations that are both reliably effective and below thresholds of concern for QT and PR interval prolongation could not be determined.
- ADVERSE REACTIONS
- The following adverse reactions are discussed in greater detail in other sections of the labeling:
- PR Interval Prolongation [see WARNINGS AND PRECAUTIONS (5.3)]
- QT Interval Prolongation [see WARNINGS AND PRECAUTIONS (5.4)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- QTcS interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once daily) controlled crossover study in 59 healthy adults, with ECG measurements on Day 3. The maximum mean (95% upper confidence bound) differences in QTcS interval from placebo after baseline-correction were 18.9 (22.0) and 30.2 (33.4) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily of INVIRASE/ritonavir, respectively. There is a delayed effect between QTc interval change and drug concentrations, with the maximum placebo-adjusted baseline-corrected QTcS observed at about 12-20 hours post-dose. INVIRASE/ritonavir 1500/100 mg twice daily resulted in a Day 3 mean Cmax of INVIRASE approximately 40% higher than the mean Cmax observed on Day 3 with the approved therapeutic dose in healthy volunteers (within the same study). QTcS in this study was QT/RR0.319 for males and QT/RR0.337 for females, which are similar to Fridericia's correction (QTcF=QT/RR0.3333).
- PR and QRS interval prolongations were also noted in subjects receiving INVIRASE/ritonavir in the same study on Day 3. The maximum mean (95% upper confidence bound) difference from placebo in the PR interval after baseline-correction were 28.6 (31.6) and 38.4 (41.4) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily saquinavir/ritonavir respectively. The maximum mean (95% upper confidence bound) difference from placebo in QRS interval after baseline correction were 2.9 (3.9) and 4.4 (5.3) ms for 1000/100 mg twice daily and supratherapeutic 1500/100 mg twice daily INVIRASE/ritonavir respectively. In this study using healthy subjects, PR interval prolongation of > 200 ms was also observed in 40% and 47% of subjects receiving INVIRASE/ritonavir 1000/100 mg bid and 1500/100 mg bid, respectively, on Day 3. Three (3%) subjects in the active control moxifloxacin arm and 5% in the placebo arm experienced PR prolongation of > 200 ms.
- The effect of treatment initiation with a dosing regimen of INVIRASE/ritonavir 500/100 mg twice daily in combination with two nucleoside reverse transcriptase inhibitors (NRTIs) for the first 7 days of treatment followed by INVIRASE/ritonavir 1000/100 mg twice daily in combination with two NRTIs in the subsequent 7 days on QTc interval, PK, and viral load was evaluated in an open-label 2-week observational study in 23 HIV-1 infected, treatment-naive patients. ECG and PK measurements were collected on Days 3, 4, 7, 10, and 14 of treatment. Two of 21 (9%) patients across all study days had maximum QTcF change from dense predose baseline ≥ 30 msec following administration of the modified INVIRASE/ritonavir regimen and the maximum mean change from dense predose baseline in the QTcF was < 10 msec across all study days. The proportion of patients with a reported PR interval prolongation > 200 msec in this study ranged from 3/22 (14%) (Day 3) to 8/21 (38%) (Day14). These results suggest that the risk of QTc interval prolongation is reduced with the modified INVIRASE/ritonavir dosing regimen, based on a cross-study comparison to the moxifloxacin-controlled QTc study described above.
- >>c00d1607-ac36-457b-a34b-75ad74f9cf0a-3.jpeg
- [Pharmacokinetics]
- Pediatric Subjects
- Steady-state pharmacokinetic information is available from HIV-1 infected pediatric subjects from study NV20911. In this study, five subjects less than 2 years of age and 13 subjects between 2 and less than 6 years of age received 50 mg per kg saquinavir twice daily (not to exceed 1000 mg twice daily) combined with ritonavir at 3 mg/kg for subjects with body weight ranging from 5 to <15 kg or 2.5 mg per kg for subjects with body weight ranging from 15 to 40 kg (not to exceed 100 mg twice daily). For subjects unable to swallow the saquinavir mesylate capsules (hard gel), the contents of saquinavir mesylate capsules (hard gel) were mixed with sugar syrup, or sorbitol syrup (for subjects with Type I diabetes or glucose intolerance), jam, or baby formula. The mean steady state saquinavir PK parameters for pediatric subjects 2 to less than 6 years of age were: AUC0-12h 37269 ± 18232 ng∙h/mL; Ctrough 1811± 998 ng/mL; Cmax 5464± 2782 ng/mL, and day 3 exposures may be within the range of exposure associated with QT and PR prolongation [see CLINICAL PHARMACOLOGY (12.2)]. The subject number was too low and the pharmacokinetic data too variable in subjects less than 2 years to establish an appropriate dosing recommendation for this age group. Pharmacokinetic data for subjects ages 6 to 16 years were not available for comparisons with observations from NV20911, as the data from HIVNAT 017 could not be validated [see USE IN SPECIFIC POPULATIONS (8.4)].
- PATIENT COUNSELING INFORMATION
- PR and QT Interval Prolongation
- Inform patients that INVIRASE/ritonavir may produce changes in the electrocardiogram (PR interval or QT interval prolongation). Advise patients to consult their health care provider if they are experiencing symptoms such as dizziness, lightheadedness, or palpitations [see WARNINGS AND PRECAUTIONS (5.3, 5.4)].
- MEDICATION GUIDE
- INVIRASE may cause serious side effects including:
- Changes in your heart rhythm and the electrical activity of your heart. These changes may be seen on an EKG (electrocardiogram) and can lead to serious heart problems. Your risk for these problems may be higher if you:
- have a history of abnormal heart rhythm, including Congenital Long QT Syndrome, or other types of heart disease.
- take other medicines that can affect your heart rhythm during treatment with INVIRASE.
- Do not take INVIRASE with ritonavir if you have the following conditions:
- a condition called Congenital Long QT Syndrome.
- complete AV (atrioventricular) heart block and you do not have a pacemaker, or you are at risk for complete AV heart block.
- low potassium or low magnesium in your blood.
- you have had a severe allergic reaction to saquinavir, saquinavir mesylate or any of the ingredients in INVIRASE. See the end of this Medication Guide for a complete list of ingredients in INVIRASE.
- severe liver problems.
- you have taken a medicine that contains rilpivirine within the last 2 weeks.
- Before taking INVIRASE, tell your healthcare provider about all of your medical conditions, including if you:
- have any heart problems, including a condition called Congenital Long QT Syndrome.
- have diabetes.
- have liver problems, including Hepatitis B or Hepatitis C.
- a history of alcoholism.
- have hemophilia.
- are pregnant or plan to become pregnant. It is not known if INVIRASE will harm your unborn baby.
- USE IN SPECIFIC POPULATIONS
- Pediatric Use
- Steady-state saquinavir exposures observed in pediatric trials were substantially higher than historical data in adults where dose- and exposure-dependent QTc and PR prolongation were observed [see WARNINGS AND PRECAUTIONS (5.3, 5.4), CLINICAL PHARMACOLOGY (12.2, 12.3)]. Although electrocardiogram abnormalities were not reported in these pediatric trials, the trials were small and not designed to evaluate QT or PR intervals. Modeling and simulation assessment of pharmacokinetic/pharmacodynamic relationships in pediatric subjects suggest that reducing the INVIRASE dose to minimize risk of QT prolongation is likely to reduce antiviral efficacy. In addition, no clinical efficacy data are available at INVIRASE doses less than 50 mg per kg in pediatric subjects. Therefore, pediatric dose recommendations that are both reliably effective and below thresholds of concern with respect to QT and PR prolongation could not be determined.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
3
42909
Other ADRs
880
14116399
Odds Ratio = 1.122
Drug Property Information
ATC Code(s):
- J05AE01 - saquinavir mesylate
- J05AE - Protease inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:saquinavir mesylate
Active Ingredient UNII:UHB9Z3841A
Drugbank ID:DB01232
PubChem Compound:441243
CAS Number:127779-20-8
Dosage Form(s):capsule; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 1800.0 mg/day J05AE01
Chemical Structure: 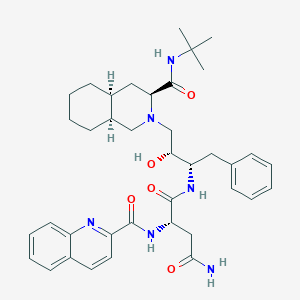

SMILE Code:
CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@H]([C@H](CC3=CC=CC=C3)NC(=O)[C@H](CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O
CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@H]([C@H](CC3=CC=CC=C3)NC(=O)[C@H](CC(=O)N)NC(=O)C4=NC5=CC=CC=C5C=C4)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.