Search for drugs:
Typing the drug name to query
CEFDINIR
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Postmarketing Experience
- The following adverse experiences and altered laboratory tests, regardless of their relationship to cefdinir, have been reported during extensive postmarketing experience, beginning with approval in Japan in 1991: shock, anaphylaxis with rare cases of fatality, facial and laryngeal edema, feeling of suffocation, serum sickness-like reactions, conjunctivitis, stomatitis, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis, erythema multiforme, erythema nodosum, acute hepatitis, cholestasis, fulminant hepatitis, hepatic failure, jaundice, increased amylase, acute enterocolitis, bloody diarrhea, hemorrhagic colitis, melena, pseudomembranous colitis, pancytopenia, granulocytopenia, leukopenia, thrombocytopenia, idiopathic thrombocytopenic purpura, hemolytic anemia, acute respiratory failure, asthmatic attack, drug-induced pneumonia, eosinophilic pneumonia, idiopathic interstitial pneumonia, fever, acute renal failure, nephropathy, bleeding tendency, coagulation disorder, disseminated intravascular coagulation, upper GI bleed, peptic ulcer, ileus, loss of consciousness, allergic vasculitis, possible cefdinir-diclofenac interaction, cardiac failure, chest pain, myocardial infarction, hypertension, involuntary movements, and rhabdomyolysis.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
8
42904
Other ADRs
2184
14115095
Odds Ratio = 1.206
Drug Property Information
ATC Code(s):
- J01DD15 - cefdinir
- J01DD - Third-generation cephalosporins
- J01D - OTHER BETA-LACTAM ANTIBACTERIALS
- J01 - ANTIBACTERIALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:cefdinir
Active Ingredient UNII:CI0FAO63WC
Drugbank ID:DB00535
PubChem Compound:6915944
CAS Number:91832-40-5
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 600.0 mg/day J01DD15
Chemical Structure: 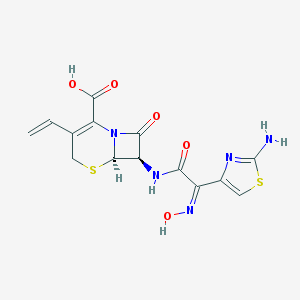

SMILE Code:
C=CC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)/C(=N\O)/C3=CSC(=N3)N)SC1)C(=O)O
C=CC1=C(N2[C@@H]([C@@H](C2=O)NC(=O)/C(=N\O)/C3=CSC(=N3)N)SC1)C(=O)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.