Search for drugs:
Typing the drug name to query
TELMISARTAN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of telmisartan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure. Decisions to include these reactions in labeling are typically based on one or more of the following factors: (1) seriousness of the reaction, (2) frequency of reporting, or (3) strength of causal connection to telmisartan.
- The most frequently spontaneously reported events include: headache, dizziness, asthenia, coughing, nausea, fatigue, weakness, edema, face edema, lower limb edema, angioneurotic edema, urticaria, hypersensitivity, sweating increased, erythema, chest pain, atrial fibrillation, congestive heart failure, myocardial infarction, blood pressure increased, hypertension aggravated, hypotension (including postural hypotension), hyperkalemia, syncope, dyspepsia, diarrhea, pain, urinary tract infection, erectile dysfunction, back pain, abdominal pain, muscle cramps (including leg cramps), myalgia, bradycardia, eosinophilia, thrombocytopenia, uric acid increased, abnormal hepatic function/liver disorder, renal impairment including acute renal failure, anemia, increased CPK, anaphylactic reaction, and tendon pain (including tendonitis, tenosynovitis), drug eruption (toxic skin eruption mostly reported as toxicoderma, rash, and urticaria), hypoglycemia (in diabetic patients), and angioedema (with fatal outcome).
- Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers, including telmisartan.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
58
42854
Other ADRs
7018
14110261
Odds Ratio = 2.722
Drug Property Information
ATC Code(s):
- C09DB04 - telmisartan
- C09DB - Angiotensin II antagonists and calcium channel blockers
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DA07 - telmisartan
- C09DA - Angiotensin II antagonists and diuretics
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09CA07 - telmisartan
- C09CA - "Angiotensin II antagonists, plain"
- C09C - "ANGIOTENSIN II ANTAGONISTS, PLAIN"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:telmisartan
Active Ingredient UNII:U5SYW473RQ
Drugbank ID:DB00966
PubChem Compound:65999
CAS Number:144701-48-4
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 40.0 mg/day C09CA07
Chemical Structure: 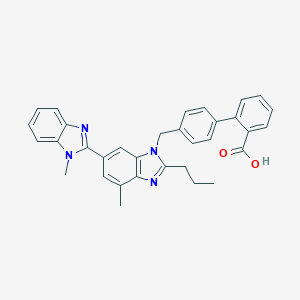

SMILE Code:
CCCC1=NC2=C(C=C(C=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C(=O)O)C5=NC6=CC=CC=C6N5C)C
CCCC1=NC2=C(C=C(C=C2N1CC3=CC=C(C=C3)C4=CC=CC=C4C(=O)O)C5=NC6=CC=CC=C6N5C)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rosuvastatin-induced rhabdomyolysis probably via CYP2C9 saturation.
[Gallelli L,Ferraro M,Spagnuolo V,Rende P,Mauro G F,De Sarro G]Drug Metabol Drug Interact.2009;24(1):83-7. PMID: 19354002
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.