Search for drugs:
Typing the drug name to query
ENTACAPONE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Other Events Reported With Dopaminergic Therapy
- The events listed below are rare events known to be associated with the use of drugs that increase dopaminergic activity, although they are most often associated with the use of direct dopamine agonists.
- Rhabdomyolysis: Cases of severe rhabdomyolysis have been reported with entacapone use. The complicated nature of these cases makes it impossible to determine what role, if any, entacapone played in their pathogenesis. Severe prolonged motor activity including dyskinesia may account for rhabdomyolysis. One case, however, included fever and alteration of consciousness. It is therefore possible that the rhabdomyolysis may be a result of the syndrome described in Hyperpyrexia and Confusion (see PRECAUTIONS, OTHER EVENTS REPORTED WITH DOPAMINERGIC THERAPY).
- Hyperpyrexia and Confusion: Cases of a symptom complex resembling the neuroleptic malignant syndrome characterized by elevated temperature, muscular rigidity, altered consciousness, and elevated CPK have been reported in association with the rapid dose reduction or withdrawal of other dopaminergic drugs. Several cases with similar signs and symptoms have been reported in association with entacapone therapy, although no information about dose manipulation is available. The complicated nature of these cases makes it difficult to determine what role, if any, entacapone may have played in their pathogenesis. No cases have been reported following the abrupt withdrawal or dose reduction of entacapone treatment during clinical studies.
- Prescribers should exercise caution when discontinuing entacapone treatment. When considered necessary, withdrawal should proceed slowly. If a decision is made to discontinue treatment with entacapone, recommendations include monitoring the patient closely and adjusting other dopaminergic treatments as needed. This syndrome should be considered in the differential diagnosis for any patient who develops a high fever or severe rigidity. Tapering entacapone has not been systematically evaluated.
- Fibrotic Complications: Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, and pleural thickening have been reported in some patients treated with ergot derived dopaminergic agents. These complications may resolve when the drug is discontinued, but complete resolution does not always occur. Although these adverse events are believed to be related to the ergoline structure of these compounds, whether other, nonergot derived drugs (e.g., entacapone) that increase dopaminergic activity can cause them is unknown. It should be noted that the expected incidence of fibrotic complications is so low that even if entacapone caused these complications at rates similar to those attributable to other dopaminergic therapies, it is unlikely that it would have been detected in a cohort of the size exposed to entacapone. Four cases of pulmonary fibrosis were reported during clinical development of entacapone; three of these patients were also treated with pergolide and one with bromocriptine. The duration of treatment with entacapone ranged from 7 to 17 months.
- Melanoma: Epidemiological studies have shown that patients with Parkinson’s disease have a higher risk (2- to approximately 6-fold higher) of developing melanoma than the general population. Whether the increased risk observed was due to Parkinson’s disease or other factors, such as drugs used to treat Parkinson’s disease, is unclear.
- For the reasons stated above, patients and providers are advised to monitor for melanomas frequently and on a regular basis when using entacapone for any indication. Ideally, periodic skin examination should be performed by appropriately qualified individuals (e.g., dermatologists).
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
42
42870
Other ADRs
2121
14115158
Odds Ratio = 6.52
Drug Property Information
ATC Code(s):
- N04BX02 - entacapone
- N04BX - Other dopaminergic agents
- N04B - DOPAMINERGIC AGENTS
- N04 - ANTI-PARKINSON DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:entacapone
Active Ingredient UNII:4975G9NM6T
Drugbank ID:DB00494
PubChem Compound:5281081
CAS Number:130929-57-6
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 1000.0 mg/day N04BX02
Chemical Structure: 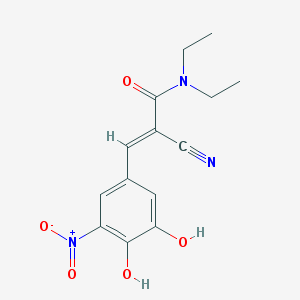

SMILE Code:
CCN(CC)C(=O)/C(=C/C1=CC(=C(C(=C1)O)O)[N+](=O)[O-])/C#N
CCN(CC)C(=O)/C(=C/C1=CC(=C(C(=C1)O)O)[N+](=O)[O-])/C#N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Levodopa + carbidopa + entacapone. Entacapone: a second look: new preparations. Parkinson's disease: a modest effect.
Prescrire Int.2005 Apr;14(76):51-4. PMID: 15875340
2: Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson's disease.
[Kaakkola S]Drugs.2000 Jun;59(6):1233-50. PMID: 10882160
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.