Search for drugs:
Typing the drug name to query
SORAFENIB
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QT Interval Prolongation
- NEXAVAR can prolong the QT/QTc interval. QT/QTc interval prolongation increases the risk for ventricular arrhythmias.
- Avoid NEXAVAR in patients with congenital long QT syndrome. Monitor electrolytes and electrocardiograms in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, including Class Ia and III antiarrhythmics. Correct electrolyte abnormalities (magnesium, potassium, calcium). Interrupt NEXAVAR if QTc interval is greater than 500 milliseconds or for an increase from baseline of 60 milliseconds or greater [see Clinical Pharmacology (12.2)].
- DRUG INTERACTIONS
- Drugs That Prolong the QT Interval
- NEXAVAR is associated with QTc interval prolongation. Avoid coadministration of NEXAVAR with medicinal products with a known potential to prolong QT/QTc interval [see Warnings and Precautions (5.9), Clinical Pharmacology (12.2)].
- DOSAGE AND ADMINISTRATION
- >>b50667e4-5ebc-4968-a646-d605058dbef0.jpeg
- ADVERSE REACTIONS
- The following clinically significant adverse reactions are discussed elsewhere in the labeling:•
- Cardiovascular events [see Warnings and Precautions (5.1)]
- Hemorrhage [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Dermatologic toxicities [see Warnings and Precautions (5.4)]
- Gastrointestinal perforation [see Warnings and Precautions (5.5)]
- QT interval prolongation [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.2)]
- Drug-induced liver injury [see Warnings and Precautions (5.10)]
- Impairment of TSH suppression in DTC [see Warnings and Precautions (5.12)]
- [Additional Data from Multiple Clinical Trials]
- Cardiovascular: Common: congestive heart failure*†, myocardial ischemia and/or infarction Uncommon: hypertensive crisis* Rare: QT prolongation*
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- The effect of NEXAVAR 400 mg twice daily on the QTc interval was evaluated in a multi-center, open-label, non-randomized trial in 53 patients with advanced cancer. No large changes in the mean QTc intervals (that is, >20 ms) from baseline were detected in the trial. After one 28-day treatment cycle, the largest mean QTc interval change of 8.5 ms (upper bound of two-sided 90% confidence interval, 13.3 ms) was observed at 6 hours post-dose on day 1 of cycle 2 [see Warnings and Precautions (5.9), Drug Interactions (7.3)].
- PATIENT COUNSELING INFORMATION
- QT Interval Prolongation
- Inform patients with a history of prolonged QT interval that NEXAVAR can worsen the condition [see Warnings and Precautions (5.9) and Clinical Pharmacology (12.2)].
- NEXAVAR may cause serious side effects, including:
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life-threatening. Your healthcare provider may do tests during your treatment with NEXAVAR to check the levels of potassium, magnesium, and calcium in your blood, and check the electrical activity of your heart with an electrocardiogram (ECG). Tell your healthcare provider right away if you feel faint, lightheaded, dizzy or feel your heart beating irregularly or fast during your treatment with NEXAVAR.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
62
42850
Other ADRs
22369
14094910
Odds Ratio = 0.912
Drug Property Information
ATC Code(s):
- L01XE05 - sorafenib
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:sorafenib
Active Ingredient UNII:9ZOQ3TZI87
Drugbank ID:DB00398
PubChem Compound:216239
CAS Number:284461-73-0
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 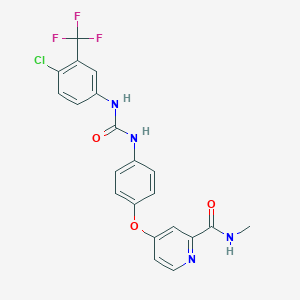

SMILE Code:
CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F
CNC(=O)C1=NC=CC(=C1)OC2=CC=C(C=C2)NC(=O)NC3=CC(=C(C=C3)Cl)C(F)(F)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Treatment compliance and severe adverse events limit the use of tyrosine kinase inhibitors in refractory thyroid cancer.
[Chrisoulidou Alexandra,Mandanas Stylianos,Margaritidou Efterpi,Mathiopoulou Lemonia,Boudina Maria,Georgopoulos Konstantinos,Pazaitou-Panayiotou Kalliopi]Onco Targets Ther.2015 Sep 3;8:2435-42. doi: 10.2147/OTT.S86322. eCollection 2015. PMID: 26366098
2: A case of rhabdomyolysis related to sorafenib treatment for advanced hepatocellular carcinoma.
[Tsuji Kunihiro,Takemura Kenichi,Minami Keisuke,Teramoto Ryota,Nakashima Keisuke,Yamada Shinya,Doyama Hisashi,Oiwake Hisanori,Hasatani Kenkou]Clin J Gastroenterol.2013 Jun;6(3):255-7. doi: 10.1007/s12328-013-0381-2. Epub 2013 May 17. PMID: 23766825
3: Sorafenib: muscle wasting.
Prescrire Int.2011 Dec;20(122):296-7. PMID: 22216546
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.