Search for drugs:
Typing the drug name to query
DIDANOSINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of didanosine. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These reactions have been chosen for inclusion due to their seriousness, frequency of reporting, causal connection to didanosine, or a combination of these factors.
- Blood and Lymphatic System Disorders - anemia, leukopenia, and thrombocytopenia.
- Body as a Whole - abdominal pain, alopecia, anaphylactoid reaction, asthenia, chills/fever, pain.
- Digestive Disorders - anorexia, dyspepsia, and flatulence.
- Exocrine Gland Disorders - pancreatitis (including fatal cases) [see WARNINGS AND PRECAUTIONS (5.1)], sialoadenitis, parotid gland enlargement, dry mouth, and dry eyes.
- Hepatobiliary Disorders - symptomatic hyperlactatemia/lactic acidosis and hepatic steatosis [see WARNINGS AND PRECAUTIONS (5.2)]; non-cirrhotic portal hypertension [see WARNINGS AND PRECAUTIONS (5.4)]; hepatitis and liver failure.
- Metabolic Disorders - diabetes mellitus, elevated serum alkaline phosphatase level, elevated serum amylase level, elevated serum gamma-glutamyltransferase level, elevated serum uric acid level, hypoglycemia, and hyperglycemia.
- Musculoskeletal Disorders - myalgia (with or without increases in creatine kinase), rhabdomyolysis including acute renal failure and hemodialysis, arthralgia, and myopathy.
- Ophthalmologic Disorders - retinal depigmentation and optic neuritis [see WARNINGS AND PRECAUTIONS (5.6)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
18
42894
Other ADRs
2754
14114525
Odds Ratio = 2.151
Drug Property Information
ATC Code(s):
- J05AF02 - didanosine
- J05AF - Nucleoside and nucleotide reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:didanosine
Active Ingredient UNII:K3GDH6OH08
Drugbank ID:DB00900
PubChem Compound:50599
CAS Number:69655-05-6
Dosage Form(s):capsule, delayed release
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day J05AF02
Chemical Structure: 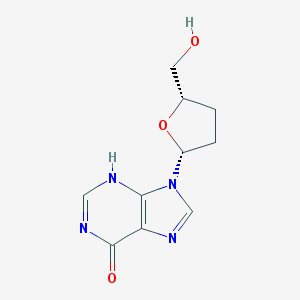

SMILE Code:
C1C[C@@H](O[C@@H]1CO)N2C=NC3=C2NC=NC3=O
C1C[C@@H](O[C@@H]1CO)N2C=NC3=C2NC=NC3=O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Severe lactic acidosis in HIV-infected patients treated with nucleosidic reverse transcriptase analogs: a report of 9 cases].
[Bonnet F,Bonarek M,Abridj A,Mercié P,Dupon M,Gemain M-C,Malvy D,Bernard N,Pellegrin J-L,Morlat P,Beylot J]Rev Med Interne.2003 Jan;24(1):11-6. PMID: 12614853
2: Acute rhabdomyolysis in patients infected by human immunodeficiency virus.
[Chariot P,Ruet E,Authier F J,Lévy Y,Gherardi R]Neurology.1994 Sep;44(9):1692-6. PMID: 7936298
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.