Search for drugs:
Typing the drug name to query
PANTOPRAZOLE SODIUM
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of pantoprazole sodium delayed-release tablets. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- These adverse reactions are listed below by body system:
- General Disorders and Administration Conditions: asthenia, fatigue, malaise
- Hematologic: pancytopenia, agranulocytosis
- Hepatobiliary Disorders: hepatocellular damage leading to jaundice and hepatic failure
- Immune System Disorders: anaphylaxis (including anaphylactic shock), systemic lupus erythematosus
- Infections and Infestations: Clostridium difficile associated diarrhea
- Investigations: weight changes
- Metabolism and Nutritional Disorders: hyponatremia, hypomagnesemia
- Musculoskeletal Disorders: rhabdomyolysis, bone fracture
- Nervous: ageusia, dysgeusia
- Psychiatric Disorders: hallucination, confusion, insomnia, somnolence
- Renal and Urinary Disorders: interstitial nephritis
- Skin and Subcutaneous Tissue Disorders: severe dermatologic reactions (some fatal), including erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis (TEN, some fatal), and angioedema (Quincke's edema) and cutaneous lupus erythematosus
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
54
42858
Other ADRs
11319
14105960
Odds Ratio = 1.571
Drug Property Information
ATC Code(s):
- A02BC02 - pantoprazole sodium
- A02BC - Proton pump inhibitors
- A02B - DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
- A02 - DRUGS FOR ACID RELATED DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
- A02BD04 - pantoprazole sodium
- A02BD - Combinations for eradication of Helicobacter pylori
- A02B - DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
- A02 - DRUGS FOR ACID RELATED DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
- A02BD11 - pantoprazole sodium
- A02BD - Combinations for eradication of Helicobacter pylori
- A02B - DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
- A02 - DRUGS FOR ACID RELATED DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:pantoprazole sodium
Active Ingredient UNII:6871619Q5X
Drugbank ID:DB00213
PubChem Compound:4679
CAS Number:102625-70-7
Dosage Form(s):tablet, delayed release
Route(s) Of Administrator:oral
Daily Dose:
- 40.0 mg/day A02BC02
Chemical Structure: 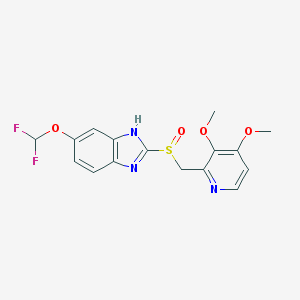

SMILE Code:
COC1=C(C(=NC=C1)CS(=O)C2=NC3=C(N2)C=C(C=C3)OC(F)F)OC
COC1=C(C(=NC=C1)CS(=O)C2=NC3=C(N2)C=C(C=C3)OC(F)F)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.