Search for drugs:
Typing the drug name to query
DILTIAZEM HYDROCHLORIDE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- PRECAUTIONS
- Drug Interactions
- Statins. Diltiazem is an inhibitor of CYP3A4 and has been shown to increase significantly the AUC of some statins. The risk of myopathy and rhabdomyolysis with statins metabolized by CYP3A4 may be increased with concomitant use of diltiazem. When possible, use a non-CYP3A4-metabolized statin together with diltiazem; otherwise, dose adjustments for both diltiazem and the statin should be considered along with close monitoring for signs and symptoms of any statin related adverse events.
- In a healthy volunteer cross-over study (N=10), co-administration of a single 20 mg dose of simvastatin at the end of a 14 day regimen with 120 mg BID diltiazem SR resulted in a 5-fold increase in mean simvastatin AUC versus simvastatin alone. Subjects with increased average steady-state exposures of diltiazem showed a greater fold increase in simvastatin exposure. Computer-based simulations showed that at a daily dose of 480 mg of diltiazem, an 8- to 9-fold mean increase in simvastatin AUC can be expected. If co-administration of simvastatin with diltiazem is required, limit the daily doses of simvastatin to 10 mg and diltiazem to 240 mg.
- In a ten-subject randomized, open label, 4-way cross-over study, co-administration of diltiazem (120 mg BID diltiazem SR for 2 weeks) with a single 20 mg dose of lovastatin resulted in 3- to 4-fold increase in mean lovastatin AUC and Cmax versus lovastatin alone. In the same study, there was no significant change in 20 mg single dose pravastatin AUC and Cmax during diltiazem coadministration. Diltiazem plasma levels were not significantly affected by lovastatin or pravastatin.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
91
42821
Other ADRs
7187
14110092
Odds Ratio = 4.173
Drug Property Information
ATC Code(s):
- C05AE03 - diltiazem hydrochloride
- C05AE - Muscle relaxants
- C05A - AGENTS FOR TREATMENT OF HEMORRHOIDS AND
- C05 - VASOPROTECTIVES
- C - CARDIOVASCULAR SYSTEM
- C08DB01 - diltiazem hydrochloride
- C08DB - Benzothiazepine derivatives
- C08D - SELECTIVE CALCIUM CHANNEL BLOCKERS WITH DIRECT CARDIAC EFFECTS
- C08 - CALCIUM CHANNEL BLOCKERS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:diltiazem hydrochloride
Active Ingredient UNII:OLH94387TE
Drugbank ID:DB00343
PubChem Compound:39186
CAS Number:42399-41-7
Dosage Form(s):capsule, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 240.0 mg/day C08DB01
Chemical Structure: 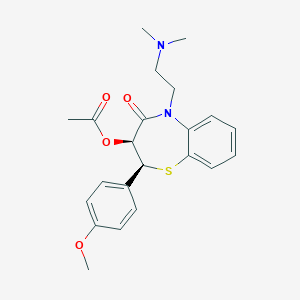

SMILE Code:
CC(=O)O[C@@H]1[C@@H](SC2=CC=CC=C2N(C1=O)CCN(C)C)C3=CC=C(C=C3)OC
CC(=O)O[C@@H]1[C@@H](SC2=CC=CC=C2N(C1=O)CCN(C)C)C3=CC=C(C=C3)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.