Search for drugs:
Typing the drug name to query
SULFASALAZINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Hypersensitivity reactions
- erythema multiforme (Stevens-Johnson syndrome), exfoliative dermatitis, epidermal necrolysis (Lyell’s syndrome) with corneal damage, drug rash with eosinophilia and systemic symptoms (DRESS), anaphylaxis, serum sickness syndrome, interstitial lung disease, pneumonitis with or without eosinophilia, vasculitis, fibrosing alveolitis, pleuritis, pericarditis with or without tamponade, allergic myocarditis, polyarteritis nodosa, lupus erythematosus-like syndrome, hepatitis and hepatic necrosis with or without immune complexes, fulminant hepatitis, sometimes leading to liver transplantation, parapsoriasis varioliformis acuta (Mucha-Haberman syndrome), rhabdomyolysis, photosensitization, arthralgia, periorbital edema, conjunctival and scleral injection, and alopecia.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
15
42897
Other ADRs
5665
14111614
Odds Ratio = 0.872
Drug Property Information
ATC Code(s):
- A07EC01 - sulfasalazine
- A07EC - Aminosalicylic acid and similar agents
- A07E - INTESTINAL ANTIINFLAMMATORY AGENTS
- A07 - "ANTIDIARRHEALS, INTESTINAL ANTIINFLAMMATORY/ANTIINFECTIVE "
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:sulfasalazine
Active Ingredient UNII:3XC8GUZ6CB
Drugbank ID:DB00795
PubChem Compound:5359476
CAS Number:599-79-1
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 2000.0 mg/day A07EC01
Chemical Structure: 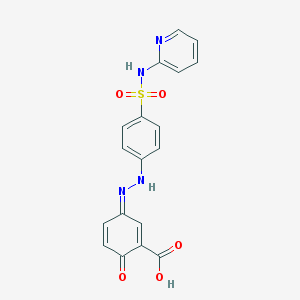

SMILE Code:
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N/N=C\3/C=CC(=O)C(=C3)C(=O)O
C1=CC=NC(=C1)NS(=O)(=O)C2=CC=C(C=C2)N/N=C\3/C=CC(=O)C(=C3)C(=O)O
Reference
COHORT STUDY:
1: Efficacy and safety of sulfasalazine in patients with chronic idiopathic urticaria.
[Orden RA, Timble H, Saini SS, Ann Allergy Asthma Immunol. 2014 Jan;112(1):64-70.]ABSTRACT
BACKGROUND: There are limited data regarding alternative treatments for antihistamine refractory chronic idiopathic urticaria (CIU). Patients with recalcitrant skin disease often cannot gain satisfactory symptom control with standard therapies and may require prolonged courses of oral corticosteroids. There is a lack of information describing the degree and duration of sulfasalazine's efficacy, the frequency and nature of adverse reactions, and the appropriate safety monitoring parameters.
OBJECTIVE: To present a case series detailing the efficacy and safety of sulfasalazine therapy in patients with CIU.
METHODS: A retrospective chart review was conducted of 39 patients with sulfasalazine-treated CIU evaluated at Johns Hopkins Asthma and Allergy Center from October 2007 to March 2012. Eight patients were excluded from the final analysis.
RESULTS: Twenty-six patients (83.9%) showed an improvement in symptoms within the first 3 months, with 51.6% of patients (n = 16) becoming asymptomatic within the first 6 months of starting sulfasalazine. Eleven patients (35.4%) achieved complete relief of symptoms after tapering off sulfasalazine therapy. Five of the 31 patients (16.1%) failed treatment, defined as worsening symptoms and pursuit of an alternative therapy. Six of 31 patients (19.4%) had a modified course of sulfasalazine therapy owing to abnormal hematologic parameters. Serious adverse events leading to drug discontinuation occurred in 6.5% of patients (n = 2) and included a patient with drug-induced leukopenia and one with rhabdomyolysis.
CONCLUSION: Sulfasalazine is a highly effective treatment for patients with antihistamine resistant CIU. The frequency of adverse events leading to an alteration of sulfasalazine treatment supports the need for close monitoring of these patients.
PMID: 24331396
OBJECTIVE: To present a case series detailing the efficacy and safety of sulfasalazine therapy in patients with CIU.
METHODS: A retrospective chart review was conducted of 39 patients with sulfasalazine-treated CIU evaluated at Johns Hopkins Asthma and Allergy Center from October 2007 to March 2012. Eight patients were excluded from the final analysis.
RESULTS: Twenty-six patients (83.9%) showed an improvement in symptoms within the first 3 months, with 51.6% of patients (n = 16) becoming asymptomatic within the first 6 months of starting sulfasalazine. Eleven patients (35.4%) achieved complete relief of symptoms after tapering off sulfasalazine therapy. Five of the 31 patients (16.1%) failed treatment, defined as worsening symptoms and pursuit of an alternative therapy. Six of 31 patients (19.4%) had a modified course of sulfasalazine therapy owing to abnormal hematologic parameters. Serious adverse events leading to drug discontinuation occurred in 6.5% of patients (n = 2) and included a patient with drug-induced leukopenia and one with rhabdomyolysis.
CONCLUSION: Sulfasalazine is a highly effective treatment for patients with antihistamine resistant CIU. The frequency of adverse events leading to an alteration of sulfasalazine treatment supports the need for close monitoring of these patients.
OTHER REFERENCE(S):
1: Efficacy and safety of sulfasalazine in patients with chronic idiopathic urticaria.
[Orden Roy Anthony,Timble Hersha,Saini Sarbjit S]Ann Allergy Asthma Immunol.2014 Jan;112(1):64-70. doi: 10.1016/j.anai.2013.09.028. Epub 2013 Nov 9. PMID: 24331396
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.