Search for drugs:
Typing the drug name to query
FENOFIBRATE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Concomitant HMG-CoA Reductase Inhibitors
- The combined use of Antara and HMG-CoA reductase inhibitors should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination.
- In a single-dose drug interaction study in 23 healthy adults the concomitant administration of fenofibrate and pravastatin resulted in no clinically important difference in the pharmacokinetics of fenofibric acid, pravastatin, or its active metabolite 3α-hydroxy iso-pravastatin when compared to either drug given alone.
- The combined use of fibric acid derivatives and HMG-CoA reductase inhibitors has been associated, in the absence of a marked pharmacokinetic interaction, in numerous case reports, with rhabdomyolysis, markedly elevated creatine kinase (CK) levels and myoglobinuria, leading in a high proportion of cases to acute renal failure.
- The use of fibrates alone, including Antara may occasionally be associated with myositis, myopathy, or rhabdomyolysis. Patients receiving Antara and complaining of muscle pain, tenderness, or weakness should have prompt medical evaluation for myopathy, including serum creatine kinase level determination. If myopathy/myositis is suspected or diagnosed, Antara therapy should be stopped.
- PRECAUTIONS
- Initial Therapy: Laboratory studies should be done to ascertain that the lipid levels are consistently abnormal before instituting Antara therapy. Every attempt should be made to control serum lipids with appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that are contributing to the lipid abnormalities. Medications known to exacerbate hypertriglyceridemia (beta-blockers, thiazides, estrogens) should be discontinued or changed if possible prior to consideration of triglyceride-lowering drug therapy.
- Continued Therapy: Periodic determination of serum lipids should be obtained during initial therapy in order to establish the lowest effective dose of Antara. Therapy should be withdrawn in patients who do not have an adequate response after two months of treatment with the maximum recommended dose of 130 mg per day.
- Pancreatitis: Pancreatitis has been reported in patients taking fenofibrate, gemfibrozil, and clofibrate. This occurrence may represent a failure of efficacy in patients with severe hypertriglyceridemia, a direct drug effect, or a secondary phenomenon mediated through biliary tract stone or sludge formation with obstruction of the common bile duct.
- Hypersensitivity Reactions: Acute hypersensitivity reactions including severe skin rashes requiring patient hospitalization and treatment with steroids have occurred very rarely during treatment with fenofibrate, including rare spontaneous reports of Stevens-Johnson syndrome, and toxic epidermal necrolysis. Urticaria was seen in 1.1 vs 0%, and rash in 1.4 vs 0.8% of fenofibrate and placebo patients, respectively, in controlled trials.
- Hematologic Changes: Mild to moderate hemoglobin, hematocrit, and white blood cell decreases have been observed in patients following initiation of fenofibrate therapy. However, these levels stabilize during long-term administration. Extremely rare spontaneous reports of thrombocytopenia and agranulocytosis have been received during post-marketing surveillance outside of the U.S. Periodic blood counts are recommended during the first 12 months of Antara administration.
- Skeletal Muscle: The use of fibrates alone, including Antara, may occasionally be associated with myopathy. Treatment with drugs of the fibrate class has been associated on rare occasions with rhabdomyolysis, usually in patients with impaired renal function. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevations of creatine phosphokinase (CPK) levels.
- Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and fenofibrate therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
372
42540
Other ADRs
5717
14111562
Odds Ratio = 21.586
Drug Property Information
ATC Code(s):
- C10AB11 - fenofibrate
- C10AB - Fibrates
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BA04 - fenofibrate
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BA03 - fenofibrate
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10AB05 - fenofibrate
- C10AB - Fibrates
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:fenofibrate
Active Ingredient UNII:U202363UOS
Drugbank ID:DB01039
PubChem Compound:3339
CAS Number:49562-28-9
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
- 200.0 mg/day C10AB05
Chemical Structure: 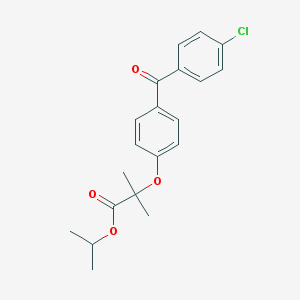

SMILE Code:
CC(C)OC(=O)C(C)(C)OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl
CC(C)OC(=O)C(C)(C)OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Efficacy and safety of fenofibrate as an add-on in patients with elevated triglyceride despite receiving statin treatment.
[Zhao Shuiping,Wang Fang,Dai Yangyang,Lin Ling,Tong Qiguang,Liao Yuhua,Yin Yuehui,Wang Guang,Yan Yafei,Li Xiaodong,Wang Daowen,Wei Ping,Cheng Xingbo,Xie Qiang,Sun Yuemin,Fu Guosheng,Huang Hongman,Dong Yugang,Liu Jianxiong,Yan Jianling,Yan Li,Cui Shiwei,Liu Xuebo,Li Zhaoping,Chen Hong,Hu Taohong,Gong Hui]Int J Cardiol.2016 Oct 15;221:832-6. doi: 10.1016/j.ijcard.2016.06.234. Epub 2016 Jun 29. PMID: 27434354
2: A 68-year old male presenting with rhabdomyolysis-associated acute kidney injury following concomitant use of elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate and pravastatin/fenofibrate: a case report.
[Suttels Veronique,Florence Eric,Leys John,Vekemans Marc,Van den Ende Jef,Vlieghe Erika,Kenyon Chris]J Med Case Rep.2015 Sep 8;9:190. doi: 10.1186/s13256-015-0671-z. PMID: 26347243
3: Evaluation of the Pharmacokinetics and Renal Excretion of Simeprevir in Subjects with Renal Impairment.
[Ouwerkerk-Mahadevan Sivi,Beumont-Mauviel Maria,Mortier Steven,Peeters Monika,Verloes Rene,Truyers Carla,Mannens Geert,Wynant Inneke,Simion Alexandru]Drugs R D.2015 Sep;15(3):261-70. doi: 10.1007/s40268-015-0101-0. PMID: 26248593
4: Safety considerations with fenofibrate/simvastatin combination.
[Filippatos Theodosios D,Elisaf Moses S]Expert Opin Drug Saf.2015;14(9):1481-93. doi: 10.1517/14740338.2015.1056778. Epub 2015 Jul 3. PMID: 26134595
5: [The combinations of statins and fibrates: pharmacokinetic and clinical implications].
[González Santos Pedro]Clin Investig Arterioscler.2014 Jul;26 Suppl 1:7-11. doi: 10.1016/S0214-9168(14)70019-1. PMID: 25043540
6: [Rhabdomyolysis secondary to simvastatin and phenofibrate].
[Forcadell-Peris M J,de Diego-Cabanes C]Semergen.2014 May-Jun;40(4):e91-4. doi: 10.1016/j.semerg.2014.01.007. Epub 2014 Apr 24. PMID: 24768027
7: Fenofibrate-induced rhabdomyolysis in a patient with stage 4 chronic renal failure due to diabetes mellitus.
[Soyoral Yasemin Usul,Canbaz Esra Turan,Erdur Mehmet Fatih,Emre Habib,Begenik Huseyin,Erkoc Reha]J Pak Med Assoc.2012 Aug;62(8):849-51. PMID: 23862266
8: A case of rhabdomyolysis complicated with acute renal failure after resumption of fenofibrate therapy: a first report.
[Kiskac Muharrem,Zorlu Mehmet,Cakirca Mustafa,Karatoprak Cumali,Peru Celalettin,Erkoc Reha,Yavuz Erdinc]Indian J Pharmacol.2013 May-Jun;45(3):305-6. doi: 10.4103/0253-7613.111912. PMID: 23833382
9: Risk of hospitalized rhabdomyolysis associated with lipid-lowering drugs in a real-world clinical setting.
[Cziraky Mark J,Willey Vincent J,McKenney James M,Kamat Siddhesh A,Fisher Maxine D,Guyton John R,Jacobson Terry A,Davidson Michael H]J Clin Lipidol.2013 Mar-Apr;7(2):102-8. doi: 10.1016/j.jacl.2012.06.006. Epub 2012 Jul 3. PMID: 23415428
10: Fenofibrate therapy in carnitine palmitoyl transferase type 2 deficiency.
[Hamilton-Craig I,Yudi M,Johnson L,Jayasinghe R]Case Rep Med.2012;2012:163173. doi: 10.1155/2012/163173. Epub 2012 Dec 3. PMID: 23326270
11: Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia.
[Guo Jinrui,Meng Fanbo,Ma Ning,Li Chunhua,Ding Zhenjiang,Wang Hong,Hou Ruitian,Qin Yingjie]Am J Cardiol.2012 Nov 1;110(9):1296-301. doi: 10.1016/j.amjcard.2012.06.050. Epub 2012 Jul 27. PMID: 22840347
12: Pravastatin and fenofibrate in combination (Pravafenix(®)) for the treatment of high-risk patients with mixed hyperlipidemia.
[Farnier Michel]Expert Rev Cardiovasc Ther.2012 May;10(5):565-75. doi: 10.1586/erc.12.37. PMID: 22651832
13: Increased toxicity when fibrates and statins are administered in combination--a metabolomics approach with rats.
[Strauss V,Mellert W,Wiemer J,Leibold E,Kamp H,Walk T,Looser R,Prokoudine A,Fabian E,Krennrich G,Herold M,van Ravenzwaay B]Toxicol Lett.2012 Jun 1;211(2):187-200. doi: 10.1016/j.toxlet.2012.03.798. Epub 2012 Mar 30. PMID: 22484644
14: Safety of a fixed-dose combination of fenofibrate/pravastatin 160 mg/40 mg in patients with mixed hyperlipidaemia: a pooled analysis from a database of clinical trials.
[Farnier Michel,Marcereuil David,De Niet Sophie,Ducobu Jean,Steinmetz Armin,Retterstøl Kjetil,Bryniarski Leszek,Császár Albert,Vanderbist Francis]Clin Drug Investig.2012 Apr 1;32(4):281-91. doi: 10.2165/11630820-000000000-00000. PMID: 22350498
15: Non-lipid effects of rosuvastatin-fenofibrate combination therapy in high-risk Asian patients with mixed hyperlipidemia.
[Lee Sang-Hak,Cho Kyoung-Im,Kim Jang-Young,Ahn Young Keun,Rha Seung-Woon,Kim Yong-Jin,Choi Yun-Seok,Choi Si Wan,Jeon Dong Woon,Min Pil-Ki,Choi Dong-Ju,Baek Sang Hong,Kim Kwon Sam,Byun Young Sup,Jang Yangsoo]Atherosclerosis.2012 Mar;221(1):169-75. doi: 10.1016/j.atherosclerosis.2011.12.042. Epub 2012 Jan 5. PMID: 22269152
16: Fenofibrate-induced rhabdomyolysis in a patient with chronic renal failure due to nephrotic syndrome: a rare case report.
[Erdur Fatih Mehmet,Soyoral Yasemin Usul,Emre Habib,Begenik Huseyin,Canbaz Esra Turan,Erkoc Reha]Clin Biochem.2012 Jan;45(1-2):162-4. doi: 10.1016/j.clinbiochem.2011.09.025. Epub 2011 Oct 14. PMID: 22019952
17: Rhabdomyolysis associated with fenofibrate monotherapy in a patient with chronic myelogenous leukemia.
[Kato Kazuya,Nagase Astushi,Matsuda Minoru,Kato Yurina,Onodera Kazuhiko,Kawakami Takako,Higuchi Mineko,Iwasaki Yoshiaki,Taniguchi Masahiko,Furukawa Hiroyuki]Case Rep Gastroenterol.2011 May;5(2):492-6. doi: 10.1159/000331559. Epub 2011 Aug 27. PMID: 21960954
18: Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population.
[Amend Kandace L,Landon Joan,Thyagarajan Veena,Niemcryk Steve,McAfee Andrew]Ann Pharmacother.2011 Oct;45(10):1230-9. doi: 10.1345/aph.1Q110. Epub 2011 Sep 13. PMID: 21917557
19: Acute renal failure due to fenofibrate monotherapy.
[Usküdar Cansu Döndü,Yaşar Nazife Sule,Korkmaz Cengiz]Anadolu Kardiyol Derg.2011 Jun;11(4):371-2. doi: 10.5152/akd.2011.092. Epub 2011 May 18. PMID: 21592934
20: Rhabdomyolysis in a Prostate Cancer Patient Taking Ketoconazole and Simvastatin: Case Report and Review of the Literature.
[Watkins Jack L,Atkinson Bradley J,Pagliaro Lance C]Ann Pharmacother.2011 Feb;45(2):e9. doi: 10.1345/aph.1P433. PMID: 21304039
21: Pharmacoepidemiology safety study of fibrate and statin concomitant therapy.
[Enger Cheryl,Gately Robert,Ming Eileen E,Niemcryk Steve J,Williams Laura,McAfee Andrew T]Am J Cardiol.2010 Dec 1;106(11):1594-601. doi: 10.1016/j.amjcard.2010.07.041. Epub 2010 Oct 14. PMID: 21094360
22: Rhabdomyolysis-induced acute renal failure following fenofibrate therapy: a case report and literature review.
[Danis Ramazan,Akbulut Sami,Ozmen Sehmus,Arikan Senay]Case Rep Med.2010;2010. pii: 537818. doi: 10.1155/2010/537818. Epub 2010 Jul 25. PMID: 20811485
23: Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study.
[Roth Eli M,McKenney James M,Kelly Maureen T,Setze Carolyn M,Carlson Dawn M,Gold Alex,Stolzenbach James C,Williams Laura A,Jones Peter H]Am J Cardiovasc Drugs.2010;10(3):175-86. doi: 10.2165/11533430-000000000-00000. PMID: 20524719
24: Fibrate therapy in the management of dyslipidemias, alone and in combination with statins: role of delayed-release fenofibric acid.
[Schima Susan M,Maciejewski Stephanie R,Hilleman Daniel E,Williams Mark A,Mohiuddin Syed M]Expert Opin Pharmacother.2010 Apr;11(5):731-8. doi: 10.1517/14656560903575639. PMID: 20210682
25: Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia.
[Kostapanos Michael S,Milionis Haralampos J,Elisaf Moses S]Am J Cardiovasc Drugs.2010;10(1):11-28. doi: 10.2165/13168600-000000000-00000. PMID: 20104931
26: Year two assessment of fenofibric acid and moderate-dose statin combination: a phase 3, open-label, extension study.
[Kipnes Mark S,Roth Eli M,Rhyne James M,Setze Carolyn M,Lele Aditya,Kelly Maureen T,Sleep Darryl J,Stolzenbach James C]Clin Drug Investig.2010;30(1):51-61. doi: 10.2165/11319800-000000000-00000. PMID: 19995098
27: After the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study: implications for fenofibrate.
[Sacks Frank M]Am J Cardiol.2008 Dec 22;102(12A):34L-40L. doi: 10.1016/j.amjcard.2008.09.073. PMID: 19084088
28: Rhabdomyolysis associated with fibrate therapy: review of 76 published cases and a new case report.
[Wu Jianyong,Song Yan,Li Heng,Chen Jianghua]Eur J Clin Pharmacol.2009 Dec;65(12):1169-74. doi: 10.1007/s00228-009-0723-7. Epub 2009 Sep 16. PMID: 19756555
29: Statins and fibrate target ClC-1 - from side effects to CLC pharmacology.
[Zdebik Anselm A]Br J Pharmacol.2009 Apr;156(8):1204-5. doi: 10.1111/j.1476-5381.2008.00083.x. PMID: 19751314
30: Fenofibrate-induced rhabdomyolysis in a patient with chronic kidney disease: an unusual presenting feature of hypothyroidism.
[Sousa Alessandra Alves de,Kronit Hans Stauber,Neves Francisco de Assis Rocha,Amato Angélica Amorim]Arq Bras Endocrinol Metabol.2009 Apr;53(3):383-6. PMID: 19578603
31: Fenofibrate monotherapy-induced rhabdomyolysis in a patient with type-2 diabetes.
[Cetinkaya Ramazan,Uyanik Abdullah,Yildirim Rahsan,Bilen Yusuf,Keles Mustafa]Indian J Med Sci.2008 Nov;62(11):458-9. PMID: 19265237
32: Efficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia.
[Goldberg Anne C,Bays Harold E,Ballantyne Christie M,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Am J Cardiol.2009 Feb 15;103(4):515-22. doi: 10.1016/j.amjcard.2008.10.025. Epub 2008 Dec 26. PMID: 19195513
33: Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study.
[Mohiuddin Syed M,Pepine Carl J,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Am Heart J.2009 Jan;157(1):195-203. doi: 10.1016/j.ahj.2008.08.027. Epub 2008 Nov 20. PMID: 19081418
34: Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study.
[Jones Peter H,Davidson Michael H,Kashyap Moti L,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Atherosclerosis.2009 May;204(1):208-15. doi: 10.1016/j.atherosclerosis.2008.09.027. Epub 2008 Oct 5. PMID: 18996523
35: Mitochondria, PPARs, and Cancer: Is Receptor-Independent Action of PPAR Agonists a Key?
[Scatena Roberto,Bottoni Patrizia,Giardina Bruno]PPAR Res.2008;2008:256251. doi: 10.1155/2008/256251. PMID: 18645611
36: Fenofibrate-induced acute renal failure due to massive rhabdomyolysis after coadministration of statin in two patients.
[Unal Aydin,Torun Edip,Sipahioglu Murat Hayri,Tokgoz Bulent,Kaya Mehmet Gungor,Oymak Oktay,Utas Cengiz]Intern Med.2008;47(11):1017-9. Epub 2008 Jun 2. PMID: 18520113
37: Hypothyroidism is a predisposing factor for fenofibrate-induced rhabdomyolysis--patient report and literature review.
[Satarasinghe R L,Ramesh R,Riyaaz A A A,Gunarathne P A K G,de Silva A P]Drug Metabol Drug Interact.2007;22(4):279-83. PMID: 18447003
38: Does the addition of fibrates to statin therapy have a favorable risk to benefit ratio?
[Brinton Eliot A]Curr Atheroscler Rep.2008 Feb;10(1):25-32. PMID: 18366982
39: Management of mixed dyslipidemia in patients with or at risk for cardiovascular disease: a role for combination fibrate therapy.
[Fazio Sergio]Clin Ther.2008 Feb;30(2):294-306. doi: 10.1016/j.clinthera.2008.02.004. PMID: 18343268
40: Relative safety of gemfibrozil and fenofibrate in the absence of concomitant cerivastatin use.
[Holoshitz Noa,Alsheikh-Ali Alawi A,Karas Richard H]Am J Cardiol.2008 Jan 1;101(1):95-7. Epub 2007 Nov 26. PMID: 18157972
41: Acute renal failure secondary to fenofibrate monotherapy-induced rhabdomyolysis.
[Tahmaz M,Kumbasar B,Ergen K,Ure U,Karatemiz G,Kazancioglu R]Ren Fail.2007;29(7):927-30. PMID: 17994463
42: Myopathy caused by a combination rosuvastatin and fenofibrate.
[Dedhia V,Munsi S C]J Assoc Physicians India.2007 Feb;55:152-3. PMID: 17571748
43: Safety of aggressive lipid management.
[Davidson Michael H,Robinson Jennifer G]J Am Coll Cardiol.2007 May 1;49(17):1753-62. Epub 2007 Apr 16. PMID: 17466224
44: Safety considerations with fibrate therapy.
[Davidson Michael H,Armani Annemarie,McKenney James M,Jacobson Terry A]Am J Cardiol.2007 Mar 19;99(6A):3C-18C. Epub 2006 Dec 8. PMID: 17368275
45: Fenofibrate: a review of its use in primary dyslipidaemia, the metabolic syndrome and type 2 diabetes mellitus.
[Keating Gillian M,Croom Katherine F]Drugs.2007;67(1):121-53. PMID: 17209672
46: Effects of chronic treatment with statins and fenofibrate on rat skeletal muscle: a biochemical, histological and electrophysiological study.
[Pierno S,Didonna M P,Cippone V,De Luca A,Pisoni M,Frigeri A,Nicchia G P,Svelto M,Chiesa G,Sirtori C,Scanziani E,Rizzo C,De Vito D,Conte Camerino D]Br J Pharmacol.2006 Dec;149(7):909-19. Epub 2006 Oct 9. PMID: 17031388
47: Risk factors for statin-associated rhabdomyolysis.
[Schech Stephanie,Graham David,Staffa Judy,Andrade Susan E,La Grenade Lois,Burgess Margaret,Blough David,Stergachis Andy,Chan K Arnold,Platt Richard,Shatin Deborah]Pharmacoepidemiol Drug Saf.2007 Mar;16(3):352-8. PMID: 16892458
48: [Rhabdomyolysis induced by fenofibrate monotherapy].
[Archambeaud-Mouveroux F,Lopez S,Combes C,Lassandre S,Amaniou M,Teissier M-P,Galinat S]Rev Med Interne.2006 Jul;27(7):573-4. Epub 2006 May 3. PMID: 16716459
49: By the way, doctor. I take Pravachol to control my cholesterol, and because of high triglycerides, my doctor wants to add TriCor. But my pharmacist warns against taking both medications. If I don't take TriCor, are there alternatives?
[Komaroff Anthony L]Harv Health Lett.2005 Mar;30(5):8. PMID: 16528800
50: Fibrates in combination with statins in the management of dyslipidemia.
[Jacobson Terry A,Zimmerman Franklin H]J Clin Hypertens (Greenwich).2006 Jan;8(1):35-41; quiz 42-3. PMID: 16407687
51: [Drug combinations: statins and fibrates].
[Xavier Hermes Toros]Arq Bras Cardiol.2005 Oct;85 Suppl 5:34-5. Epub 2006 Jan 2. PMID: 16400396
52: A case of rhabdomyolysis induced acute renal failure secondary to statin-fibrate-derivative combination and occult hypothyroidism.
[Kursat S,Alici T,Colak H B]Clin Nephrol.2005 Nov;64(5):391-3. PMID: 16312269
53: A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087.
[Aberg Judith A,Zackin Robert A,Brobst Susan W,Evans Scott R,Alston Beverly L,Henry W Keith,Glesby Marshall J,Torriani Francesca J,Yang Yijun,Owens Susan I,Fichtenbaum Carl J,ACTG 5087 Study Team]AIDS Res Hum Retroviruses.2005 Sep;21(9):757-67. PMID: 16218799
54: Rhabdomyolysis with cardiac involvement and acute renal failure in a patient taking rosuvastatin and fenofibrate.
[Ireland James H E,Eggert Christoph H,Arendt Christopher J,Williams Amy W]Ann Intern Med.2005 Jun 7;142(11):949-50. PMID: 15941707
55: Simvastatin, fenofibrate, and rhabdomyolysis.
[Jacob Sindhu S,Jacob Sony,Williams Craig,Deeg Mark A]Diabetes Care.2005 May;28(5):1258. PMID: 15855605
56: Reporting rate of rhabdomyolysis with fenofibrate + statin versus gemfibrozil + any statin.
[Jones Peter H,Davidson Michael H]Am J Cardiol.2005 Jan 1;95(1):120-2. PMID: 15619408
57: Risk of adverse events with fibrates.
[Alsheikh-Ali Alawi A,Kuvin Jeffrey T,Karas Richard H]Am J Cardiol.2004 Oct 1;94(7):935-8. PMID: 15464682
58: Safety of statins: focus on clinical pharmacokinetics and drug interactions.
[Bellosta Stefano,Paoletti Rodolfo,Corsini Alberto]Circulation.2004 Jun 15;109(23 Suppl 1):III50-7. PMID: 15198967
59: [Polymyositis induced or associated with lipid-lowering drugs: five cases].
[Fauchais A-L,Iba Ba J,Maurage P,Kyndt X,Bataille D,Hachulla E,Parent D,Queyrel V,Lambert M,Michon Pasturel U,Hatron P-Y,Vanhille P,Devulder B]Rev Med Interne.2004 Apr;25(4):294-8. PMID: 15050796
60: Influence of lipid lowering fibrates on P-glycoprotein activity in vitro.
[Ehrhardt Manuela,Lindenmaier Heike,Burhenne Juergen,Haefeli Walter Emil,Weiss Johanna]Biochem Pharmacol.2004 Jan 15;67(2):285-92. PMID: 14698041
61: [Long-term hypolipidemic treatment of mixed hyperlipidemia with a combination of statins and fibrates].
[Zeman M,Zák A,Vecka M,Romaniv S]Cas Lek Cesk.2003 Aug;142(8):500-4. PMID: 14626567
62: Fenofibrate monotherapy induced rhabdomyolysis.
[Barker Billie J,Goodenough Roger R,Falko James M]Diabetes Care.2003 Aug;26(8):2482-3. PMID: 12882895
63: Cell-specific toxicity of fibrates in human embryonal rhabdomyosarcoma cells.
[Maiguma Takayoshi,Fujisaki Koji,Itoh Yoshinori,Makino Kazutaka,Teshima Daisuke,Takahashi-Yanaga Fumi,Sasaguri Toshiyuki,Oishi Ryozo]Naunyn Schmiedebergs Arch Pharmacol.2003 Mar;367(3):289-96. Epub 2003 Feb 8. PMID: 12644902
64: Optimizing bexarotene therapy for cutaneous T-cell lymphoma.
[Talpur Rakhshandra,Ward Staci,Apisarnthanarax Narin,Breuer-Mcham Joan,Duvic Madeleine]J Am Acad Dermatol.2002 Nov;47(5):672-84. PMID: 12399758
65: Cerivastatin and gemfibrozil-associated rhabdomyolysis.
[Bruno-Joyce J,Dugas J M,MacCausland O E]Ann Pharmacother.2001 Sep;35(9):1016-9. PMID: 11573847
66: Rhabdomyolysis and acute renal failure after changing statin-fibrate combinations.
[Oldemeyer J B,Lund R J,Koch M,Meares A J,Dunlay R]Cardiology.2000;94(2):127-8. PMID: 11173785
67: [Septic-toxic shock due to rhabdomyolysis in a patient treated with fenofibrate].
[Duda-Król W,Jórasz I,Dabrowska M,Polubiec A,Kusz-Rynkun A]Wiad Lek.2000;53(7-8):454-7. PMID: 11070769
68: Micronized fenofibrate: a new fibric acid hypolipidemic agent.
[Guay D R]Ann Pharmacother.1999 Oct;33(10):1083-103. PMID: 10534222
69: Fenofibrate-induced rhabdomyolysis in two dialysis patients with hypothyroidism.
[Clouâtre Y,Leblanc M,Ouimet D,Pichette V]Nephrol Dial Transplant.1999 Apr;14(4):1047-8. PMID: 10328516
70: Fenofibrate plus simvastatin therapy versus simvastatin plus cholestyramine therapy for familial hypercholesterolaemia.
[Wierzbicki A S,Lumb P J,Cheung J,Crook M A]QJM.1997 Oct;90(10):631-4. PMID: 9415344
71: [Thyroid myopathy disclosed by a fibrate].
[Schlienger J L,Goichot B,Grunenberger F,Pradignac A]Rev Med Interne.1997;18(2):169-70. PMID: 9092037
72: Currently available hypolipidaemic drugs and future therapeutic developments.
[Farmer J A,Gotto A M]Baillieres Clin Endocrinol Metab.1995 Oct;9(4):825-47. PMID: 8593127
73: [Necrotizing myopathy with antilipemic agents. Case report and review of the literature].
[Berger O,Zifko U,Jellinger K,Machacek E,Grisold W]Nervenarzt.1993 Aug;64(8):539-44. PMID: 8413753
74: [Biological myolysis during combined fenofibrate-pravastatin therapy].
[Raimondeau J,Le Marec H,Chevallier J C,Bouhour J B]Presse Med.1992 Apr 11;21(14):663-4. PMID: 1534619
75: [Side effects of fibrates (except liver and muscle)].
[Sgro C,Escousse A]Therapie.1991 Sep-Oct;46(5):351-4. PMID: 1754977
76: [Rhabdomyolysis with acute renal insufficiency: role of the association of fibrate and hypothyroidism].
[Fredenrich A,Sadoul J L,Jambou P,Vinti H]Rev Med Interne.1991 May-Jun;12(3):238. PMID: 1896719
77: Effects of gemfibrozil and other fibric acid derivatives on blood lipids and lipoproteins.
[Zimetbaum P,Frishman W H,Kahn S]J Clin Pharmacol.1991 Jan;31(1):25-37. PMID: 2045526
78: Bezafibrate. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hyperlipidaemia.
[Monk J P,Todd P A]Drugs.1987 Jun;33(6):539-76. PMID: 3301301
79: [Bezafibrate-induced myolysis and myoglobinuria in patients with impaired renal function].
[Rumpf K W,Barth M,Blech M,Kaiser H,Koop I,Arnold R,Scheler F]Klin Wochenschr.1984 Apr 16;62(8):346-8. PMID: 6727274
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.