Search for drugs:
Typing the drug name to query
TETRABENAZINE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- Tetrabenazine causes a small increase (about 8 msec) in the corrected QT (QTc) interval. QT prolongation can lead to development of torsade de pointes-type ventricular tachycardia with the risk increasing as the degree of prolongation increases [see CLINICAL PHARMACOLOGY (12.2)]. The use of tetrabenazine tablets should be avoided in combination with other drugs that are known to prolong QTc, including antipsychotic medications (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), Class 1A (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications or any other medications known to prolong the QTc interval [see Drug Interactions (7.5)].
- Tetrabenazine should also be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval [see Clinical Pharmacology (12.2)].
- DRUG INTERACTIONS
- Drugs That Cause QTc Prolongation
- Tetrabenazine causes a small prolongation of QTc (about 8 msec), concomitant use with other drugs that are known to cause QTc prolongation should be avoided, these including antipsychotic medications (e.g., chlorpromazine, haloperidol, thioridazine, ziprasidone), antibiotics (e.g., moxifloxacin), Class 1A (e.g., quinidine, procainamide) and Class III (e.g., amiodarone, sotalol) antiarrhythmic medications or any other medications known to prolong the QTc interval. Tetrabenazine should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain conditions may increase the risk for torsade de pointes or sudden death such as (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval [see Warnings and Precautions (5.8), Clinical Pharmacology (12.2)].
- ADVERSE REACTIONS
- The following serious adverse reactions are described below and elsewhere in the labeling:
- Depression and Suicidality [see Warnings and Precautions (5.1)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)]
- Akathisia, Restlessness, and Agitation [see Warnings and Precautions (5.5)]
- Parkinsonism [see Warnings and Precautions (5.6)]
- Sedation and Somnolence [see Warnings and Precautions (5.7)]
- QTc Prolongation [see Warnings and Precautions (5.8)]
- Hypotension and Orthostatic Hypotension [see Warnings and Precautions (5.9)]
- Hyperprolactinemia [see Warnings and Precautions (5.10)]
- Binding to Melanin-Containing Tissues [see Warnings and Precautions (5.11)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- QTc Prolongation
- The effect of a single 25 or 50 mg dose of tetrabenazine on the QT interval was studied in a randomized, double-blind, placebo-controlled crossover study in healthy male and female subjects with moxifloxacin as a positive control. At 50 mg, tetrabenazine caused an approximately 8 msec mean increase in QTc (90% CI: 5.0, 10.4 msec). Additional data suggest that inhibition of CYP2D6 in healthy subjects given a single 50 mg dose of tetrabenazine does not further increase the effect on the QTc interval. Effects at higher exposures to either tetrabenazine or its metabolites have not been evaluated [see Warnings and Precautions (5.8), Drug Interactions (7.5)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
6
42906
Other ADRs
6205
14111074
Odds Ratio = 0.319
Drug Property Information
ATC Code(s):
- N07XX06 - tetrabenazine
- N07XX - Other nervous system drugs
- N07X - OTHER NERVOUS SYSTEM DRUGS
- N07 - OTHER NERVOUS SYSTEM DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:tetrabenazine
Active Ingredient UNII:Z9O08YRN8O
Drugbank ID:DB04844
PubChem Compound:6018
CAS Number:58-46-8
Dosage Form(s):tablet, coated
Route(s) Of Administrator:oral
Daily Dose:
- 100.0 mg/day N07XX06
Chemical Structure: 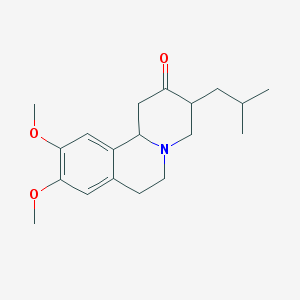

SMILE Code:
CC(C)CC1CN2CCC3=CC(=C(C=C3C2CC1=O)OC)OC
CC(C)CC1CN2CCC3=CC(=C(C=C3C2CC1=O)OC)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Severe hyperthermia during tetrabenazine therapy for tardive dyskinesia.
[Stevens E,Roman A,Houa M,Razavi D,Jaspar N]Intensive Care Med.1998 Apr;24(4):369-71. PMID: 9609418
2: Status dystonicus: the syndrome and its management.
[Manji H,Howard R S,Miller D H,Hirsch N P,Carr L,Bhatia K,Quinn N,Marsden C D,Bahtia K]Brain.1998 Feb;121 ( Pt 2):243-52. PMID: 9549503
3: Pathogenesis and treatment of neuroleptic malignant syndrome.
[Ebadi M,Pfeiffer R F,Murrin L C]Gen Pharmacol.1990;21(4):367-86. PMID: 1974219
4: Severe dystonia and myoglobinuria.
[Jankovic J,Penn A S]Neurology.1982 Oct;32(10):1195-7. PMID: 6889706
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.