Search for drugs:
Typing the drug name to query
ROTIGOTINE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Hyperpyrexia and Confusion
- A symptom complex resembling the neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, rhabdomyolysis, and/or autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in anti-Parkinsonian therapy. Therefore, it is recommended that the dose be tapered at the end of NEUPRO treatment [see DOSAGE AND ADMINISTRATION (2.4)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
9
42903
Other ADRs
3310
14113969
Odds Ratio = 0.895
Drug Property Information
ATC Code(s):
- N04BC09 - rotigotine
- N04BC - Dopamine agonists
- N04B - DOPAMINERGIC AGENTS
- N04 - ANTI-PARKINSON DRUGS
- N - NERVOUS SYSTEM
Active Ingredient:rotigotine
Active Ingredient UNII:87T4T8BO2E
Drugbank ID:DB05271
PubChem Compound:59227
CAS Number:99755-59-6
Dosage Form(s):kit; patch, extended release
Route(s) Of Administrator:transdermal
Daily Dose:
Chemical Structure: 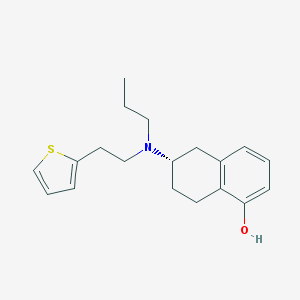

SMILE Code:
CCCN(CCC1=CC=CS1)[C@H]2CCC3=C(C2)C=CC=C3O
CCCN(CCC1=CC=CS1)[C@H]2CCC3=C(C2)C=CC=C3O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.