Search for drugs:
Typing the drug name to query
DESVENLAFAXINE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Human Experience with Overdosage
- In postmarketing experience, overdose with venlafaxine (the parent drug of desvenlafaxine) has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported events in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), sinus and ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- ECG changes
- Electrocardiograms were obtained from 1,492 desvenlafaxine treated patients with major depressive disorder and 984 placebo-treated patients in clinical studies lasting up to 8 weeks. No clinically relevant differences were observed between desvenlafaxine treated and placebo-treated patients for QT, QTc, PR, and QRS intervals. In a thorough QTc study with prospectively determined criteria, desvenlafaxine did not cause QT prolongation. No difference was observed between placebo and desvenlafaxine treatments for the QRS interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
2
42910
Other ADRs
281
14116998
Odds Ratio = 2.342
Drug Property Information
ATC Code(s):
- N06AX23 - desvenlafaxine
- N06AX - Other antidepressants
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:desvenlafaxine
Active Ingredient UNII:NG99554ANW
Drugbank ID:DB06700
PubChem Compound:125017
CAS Number:93413-62-8
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 50.0 mg/day N06AX23
Chemical Structure: 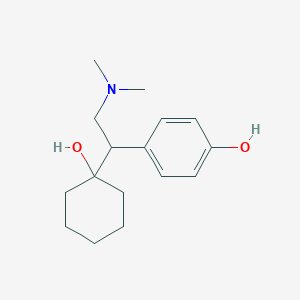

SMILE Code:
CN(C)CC(C1=CC=C(C=C1)O)C2(CCCCC2)O
CN(C)CC(C1=CC=C(C=C1)O)C2(CCCCC2)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.