Search for drugs:
Typing the drug name to query
PIMOZIDE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS
- Other
- Sudden, unexpected deaths have occurred in experimental studies of conditions other than Tourette's Disorder. These deaths occurred while patients were receiving dosages in the range of 1 mg per kg. One possible mechanism for such deaths is prolongation of the QT interval predisposing patients to ventricular arrhythmia. An electrocardiogram should be performed before pimozide treatment is initiated and periodically thereafter, especially during the period of dose adjustment.
- PRECAUTIONS
- LABORATORY TESTS
- An ECG should be done at baseline and periodically thereafter throughout the period of dose adjustment. Any indication of prolongation of QTc interval beyond an absolute limit of 0.47 seconds (children) or 0.52 seconds (adults), or more than 25% above the patient’s original baseline should be considered a basis for stopping further dose increase (see CONTRAINDICATIONS) and considering a lower dose.
- [DRUG INTERACTIONS]
- Because pimozide prolongs the QT interval of the electrocardiogram, an additive effect on QT interval would be anticipated if administered with other drugs, such as phenothiazines, tricyclic antidepressants or antiarrhythmic agents, which prolong the QT interval. Accordingly, pimozide should not be given with dofetilide, sotalol, quinidine, other Class Ia and III anti-arrhythmics, mesoridazine, thioridazine, chlorpromazine, droperidol, sparfloxacin, gatifloxacin, moxifloxacin, halofantrine, mefloquine, pentamidine, arsenic trioxide, levomethadyl acetate, dolasetron mesylate, probucol, tacrolimus, ziprasidone, or other drugs that have demonstrated QT prolongation as one of their pharmacodynamic effects. Also, the use of macrolide antibiotics in patients with prolonged QT intervals has been rarely associated with ventricular arrhythmias. Such concomitant administration should not be undertaken (see CONTRAINDICATIONS).
- Pimozide and Celexa: In a controlled study, a single dose of pimozide 2 mg coadministered with racemic citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately 10 msec compared to pimozide given alone. Racemic citalopram did not alter the mean AUC or Cmax of pimozide. The mechanism of this pharmacodynamic interaction is not known. Concomitant use of Pimozide and Celexa or Lexapro is contraindicated (See CONTRAINDICATIONS).
- CONTRAINDICATIONS
- Because pimozide prolongs the QT interval of the electrocardiogram it is contraindicated in patients with congenital long QT syndrome, patients with a history of cardiac arrhythmias, patients taking other drugs which prolong the QT interval of the electrocardiogram or patients with known hypokalemia or hypomagnesemia (see also PRECAUTIONS - Drug Interactions).
- Ventricular arrhythmias have been rarely associated with the use of macrolide antibiotics in patients with prolonged QT intervals, as might be produced by pimozide. Specifically, two sudden deaths have been reported when clarithromycin was added to ongoing pimozide therapy.Furthermore, some evidence suggests that pimozide is metabolized partly by the enzyme system cytochrome P450 3A4 (CYP 3A4). Macrolide antibiotics are inhibitors of CYP 3A4, and thus could potentially impede pimozide metabolism. For these reasons, pimozide is contraindicated in patients receiving the macrolide antibiotics clarithromycin, erythromycin, azithromycin,dirithromycin, and troleandomycin.
- ADVERSE REACTIONS
- Electrocardiographic Changes: Electrocardiographic changes have been observed in clinical trials of pimozide in Tourette’s Disorder and schizophrenia. These have included prolongation of the QT interval, flattening, notching and inversion of the T wave and the appearance of U waves. Sudden, unexpected deaths and grand mal seizure have occurred at doses above 20 mg/day.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
3
42909
Other ADRs
308
14116971
Odds Ratio = 3.205
Drug Property Information
ATC Code(s):
- N05AG02 - pimozide
- N05AG - Diphenylbutylpiperidine derivatives
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:pimozide
Active Ingredient UNII:1HIZ4DL86F
Drugbank ID:DB01100
PubChem Compound:16362
CAS Number:2062-78-4
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 4.0 mg/day N05AG02
Chemical Structure: 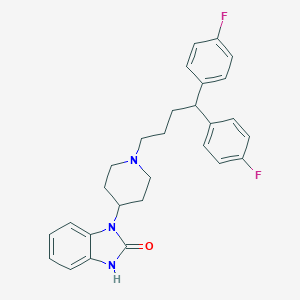

SMILE Code:
C1CN(CCC1N2C3=CC=CC=C3NC2=O)CCCC(C4=CC=C(C=C4)F)C5=CC=C(C=C5)F
C1CN(CCC1N2C3=CC=CC=C3NC2=O)CCCC(C4=CC=C(C=C4)F)C5=CC=C(C=C5)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet.2000 Jan;38(1):41-57. PMID: 10668858
2: Status dystonicus: the syndrome and its management.
[Manji H,Howard R S,Miller D H,Hirsch N P,Carr L,Bhatia K,Quinn N,Marsden C D,Bahtia K]Brain.1998 Feb;121 ( Pt 2):243-52. PMID: 9549503
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.