Search for drugs:
Typing the drug name to query
FLUVASTATIN SODIUM
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Skeletal Muscle
- Rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with LESCOL/LESCOL XL and other drugs in this class.
- LESCOL/LESCOL XL should be prescribed with caution in patients with predisposing factors for myopathy. These factors include advanced age (>65 years), renal impairment, and inadequately treated hypothyroidism.
- The risk of myopathy and/or rhabdomyolysis with statins is increased with concurrent therapy with cyclosporine, erythromycin, fibrates or niacin. Myopathy was not observed in a clinical trial in 74 patients involving patients who were treated with LESCOL/LESCOL XL together with niacin. Isolated cases of myopathy have been reported during post-marketing experience with concomitant administration of LESCOL/LESCOL XL and colchicine. No information is available on the pharmacokinetic interaction between LESCOL/LESCOL XL and colchicine.
- Uncomplicated myalgia has also been reported in LESCOL-treated patients [see Adverse Reactions (6)]. In clinical trials, uncomplicated myalgia has been observed infrequently in patients treated with LESCOL at rates indistinguishable from placebo. Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in CPK values to greater than 10 times the upper limit of normal, was <0.1% in fluvastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK.
- There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
- All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing LESCOL/LESCOL XL.
- LESCOL/LESCOL XL therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected. LESCOL/LESCOL XL therapy should also be temporarily withheld in any patient experiencing an acute or serious condition predisposing to the development of renal failure secondary to rhabdomyolysis, e.g., sepsis; hypotension; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
- ADVERSE REACTIONS
- The following serious adverse reactions are discussed in greater detail in other sections of the label:
- - Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see Warnings and Precautions (5.1)].
- - Liver Enzyme Abnormalities [see Warnings and Precautions (5.2)].
- Postmarketing Experience
- Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following effects have been reported with drugs in this class. Not all the effects listed below have necessarily been associated with fluvastatin sodium therapy.
- Musculoskeletal: muscle cramps, myalgia, myopathy, rhabdomyolysis, arthralgias, muscle spasms, muscle weakness, myositis.
- There have been rare reports of immune-mediated necrotizing myopathy associated with statin use [see Warnings and Precautions (5.1)].
- DRUG INTERACTIONS
- Gemfibrozil
- Due to an increased risk of myopathy/rhabdomyolysis when HMG-CoA reductase inhibitors are coadministered with gemfibrozil, concomitant administration of LESCOL/LESCOL XL with gemfibrozil should be avoided.
- Colchicine
- Cases of myopathy, including rhabdomyolysis, have been reported with fluvastatin coadministered with colchicine, and caution should be exercised when prescribing fluvastatin with colchicine.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
253
42659
Other ADRs
2901
14114378
Odds Ratio = 28.856
Drug Property Information
ATC Code(s):
- C10AA04 - fluvastatin sodium
- C10AA - HMG CoA reductase inhibitors
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:fluvastatin sodium
Active Ingredient UNII:PYF7O1FV7F
Drugbank ID:DB01095
PubChem Compound:1548972
CAS Number:93957-54-1
Dosage Form(s):tablet, extended release
Route(s) Of Administrator:oral
Daily Dose:
- 60.0 mg/day C10AA04
Chemical Structure: 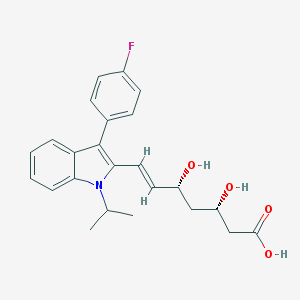

SMILE Code:
CC(C)N1C2=CC=CC=C2C(=C1/C=C/[C@@H](C[C@@H](CC(=O)O)O)O)C3=CC=C(C=C3)F
CC(C)N1C2=CC=CC=C2C(=C1/C=C/[C@@H](C[C@@H](CC(=O)O)O)O)C3=CC=C(C=C3)F
Reference
COHORT STUDY:
1: Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information.
[Chang CH, Kusama M, Ono S, Sugiyama Y, Orii T, Akazawa M, BMJ Open. 2013 Apr 11;3(4).]ABSTRACT
OBJECTIVE: To estimate the incidence of muscle toxicity in patients receiving statin therapy by examining study populations, drug exposure status and outcome definitions.
DESIGN: A retrospective cohort study.
SETTING: 16 medical facilities in Japan providing information on laboratory tests performed in and claims received by their facilities between 1 April 2004 and 31 December 2010.
PARTICIPANTS: A database representing a cohort of 35 903 adult statin (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin) users was studied. Use of interacting drugs (fibrates, triazoles, macrolides, amiodarone and ciclosporin) by these patients was determined.
MAIN OUTCOME MEASURE: Statin-associated muscle toxicity (the 'event') was identified based on a diagnosis of muscle-related disorders (myopathy or rhabdomyolysis) and/or abnormal elevation of creatine kinase (CK) concentrations. Events were excluded if the patients had CK elevation-related conditions other than muscle toxicity. Incidence rates for muscle toxicity were determined per 1000 person-years, with 95% CI determined by Poisson regression.
RESULTS: A total of 18 036 patients accounted for 42 193 person-years of statin therapy, and 43 events were identified. The incidence of muscle toxicity in the patients treated with statins was 1.02 (95% CI 0.76 to 1.37)/1000 person-years. The estimates varied when outcome definitions were modified from 0.09/1000 person-years, which met both diagnosis and CK 10× greater than the upper limit of normal range (ULN) criteria, to 2.06/1000 person-years, which met diagnosis or CK 5× ULN criterion. The incidence of muscle toxicity was also influenced by the statin therapies selected, but no significant differences were observed. Among 2430 patients (13.5%) received interacting drugs with statins, only three muscle toxicity cases were observed (incidence: 1.69/1000 person-years).
CONCLUSIONS: This database study suggested that statin use is generally well tolerated and safe; however, the risk of muscle toxicity related to the use of interacting drugs requires further exploration.
PMID: 23585384
DESIGN: A retrospective cohort study.
SETTING: 16 medical facilities in Japan providing information on laboratory tests performed in and claims received by their facilities between 1 April 2004 and 31 December 2010.
PARTICIPANTS: A database representing a cohort of 35 903 adult statin (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin) users was studied. Use of interacting drugs (fibrates, triazoles, macrolides, amiodarone and ciclosporin) by these patients was determined.
MAIN OUTCOME MEASURE: Statin-associated muscle toxicity (the 'event') was identified based on a diagnosis of muscle-related disorders (myopathy or rhabdomyolysis) and/or abnormal elevation of creatine kinase (CK) concentrations. Events were excluded if the patients had CK elevation-related conditions other than muscle toxicity. Incidence rates for muscle toxicity were determined per 1000 person-years, with 95% CI determined by Poisson regression.
RESULTS: A total of 18 036 patients accounted for 42 193 person-years of statin therapy, and 43 events were identified. The incidence of muscle toxicity in the patients treated with statins was 1.02 (95% CI 0.76 to 1.37)/1000 person-years. The estimates varied when outcome definitions were modified from 0.09/1000 person-years, which met both diagnosis and CK 10× greater than the upper limit of normal range (ULN) criteria, to 2.06/1000 person-years, which met diagnosis or CK 5× ULN criterion. The incidence of muscle toxicity was also influenced by the statin therapies selected, but no significant differences were observed. Among 2430 patients (13.5%) received interacting drugs with statins, only three muscle toxicity cases were observed (incidence: 1.69/1000 person-years).
CONCLUSIONS: This database study suggested that statin use is generally well tolerated and safe; however, the risk of muscle toxicity related to the use of interacting drugs requires further exploration.
OTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.