Search for drugs:
Typing the drug name to query
RALTEGRAVIR
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose 1.33 times the maximum approved recommended dose (and peak concentrations 1.25-fold higher than the maximum approved dose), raltegravir does not prolong the QT interval or PR interval to any clinically relevant extent.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
55
42857
Other ADRs
4918
14112361
Odds Ratio = 3.683
Drug Property Information
ATC Code(s):
- J05AX08 - raltegravir
- J05AX - Other antivirals
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR16 - raltegravir
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:raltegravir potassium
Active Ingredient UNII:43Y000U234
Drugbank ID:DB06817
PubChem Compound:54671008
CAS Number:518048-05-0
Dosage Form(s):granule, for suspension; tablet, chewable; tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 800.0 mg/day J05AX08
Chemical Structure: 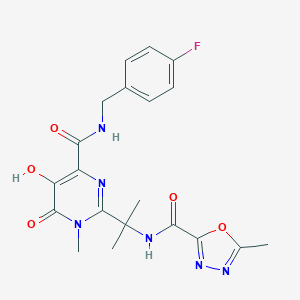

SMILE Code:
CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F
CC1=NN=C(O1)C(=O)NC(C)(C)C2=NC(=C(C(=O)N2C)O)C(=O)NCC3=CC=C(C=C3)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Muscle symptoms and creatine phosphokinase elevations in patients receiving raltegravir in clinical practice: Results from the SCOLTA project long-term surveillance.
[Madeddu Giordano,De Socio Giuseppe V L,Ricci Elena,Quirino Tiziana,Orofino Giancarlo,Carenzi Laura,Franzetti Marco,Parruti Giustino,Martinelli Canio,Vichi Francesca,Penco Giovanni,Dentone Chiara,Celesia Benedetto Maurizio,Maggi Paolo,Libertone Raffaella,Bagella Paola,Di Biagio Antonio,Bonfanti Paolo,C.I.S.A.I. Group, Italy]Int J Antimicrob Agents.2015 Mar;45(3):289-94. doi: 10.1016/j.ijantimicag.2014.10.013. Epub 2014 Nov 13. PMID: 25476452
2: Skeletal muscle toxicity in HIV-1-infected patients treated with a raltegravir-containing antiretroviral therapy: a cohort study.
[Calza Leonardo,Danese Ilaria,Colangeli Vincenzo,Vandi Giacomo,Manfredi Roberto,Girometti Nicolò,Borderi Marco,Appolloni Lucia,Puggioli Cristina,Viale Pierluigi]AIDS Res Hum Retroviruses.2014 Dec;30(12):1162-9. doi: 10.1089/aid.2014.0113. PMID: 25369244
3: No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database.
[Napoli Andrew A,Wood Jennifer J,Coumbis John J,Soitkar Amit M,Seekins Daniel W,Tilson Hugh H]J Int AIDS Soc.2014 Sep 4;17:19214. doi: 10.7448/IAS.17.1.19214. eCollection 2014. PMID: 25192857
4: [Severe or life-threatening interactions between antiretrovirals and non-HIV drugs].
[Manzardo Christian,Tuset Montserrat,Miró Jose M,Gatell Jose M]Enferm Infecc Microbiol Clin.2015 Aug-Sep;33(7):e15-30. doi: 10.1016/j.eimc.2014.02.020. Epub 2014 Jun 7. PMID: 24913990
5: Long-term efficacy and safety of raltegravir in the management of HIV infection.
[Liedtke Michelle D,Tomlin C Ryan,Lockhart Staci M,Miller Misty M,Rathbun R Chris]Infect Drug Resist.2014 Mar 18;7:73-84. doi: 10.2147/IDR.S40168. eCollection 2014. PMID: 24672249
6: Rapid onset of rhabdomyolysis after switching to a raltegravir-based antiretroviral regimen.
[Tsai Wan-Jung,Lee Susan Shin-Jung,Tsai Hung-Chin,Sy Cheng-Len,Chen Jui-Kuang,Wu Kuang-Sheng,Wang Yung-Hsin,Chen Yao-Shen]J Microbiol Immunol Infect.2016 Apr;49(2):286-8. doi: 10.1016/j.jmii.2013.02.008. Epub 2013 Apr 21. PMID: 23612027
7: Skeletal muscle toxicity associated with raltegravir-based combination antiretroviral therapy in HIV-infected adults.
[Lee Frederick J,Amin Janaki,Bloch Mark,Pett Sarah L,Marriott Debbie,Carr Andrew]J Acquir Immune Defic Syndr.2013 Apr 15;62(5):525-33. PMID: 23274936
8: Tolerability of HIV integrase inhibitors.
[Lee Frederick J,Carr Andrew]Curr Opin HIV AIDS.2012 Sep;7(5):422-8. doi: 10.1097/COH.0b013e328356682a. PMID: 22886031
9: Common adverse effects of antiretroviral therapy for HIV disease.
[Reust Carin E]Am Fam Physician.2011 Jun 15;83(12):1443-51. PMID: 21671545
10: Severe raltegravir-associated rhabdomyolysis: a case report and review of the literature.
[Croce F,Vitello P,Dalla Pria A,Riva A,Galli M,Antinori S]Int J STD AIDS.2010 Nov;21(11):783-5. doi: 10.1258/ijsa.2010.010246. PMID: 21187364
11: Severe acute renal failure associated with rhabdomyolysis during treatment with raltegravir. A call for caution.
[Masiá Mar,Enríquez Ricardo,Sirvent Ana,Gutiérrez Félix]J Infect.2010 Jul;61(2):189-90. doi: 10.1016/j.jinf.2010.04.011. Epub 2010 May 4. PMID: 20447415
12: A case of rhabdomiolysis associated with raltegravir use.
[Dori Luca,Buonomini Anna R,Viscione Magda,Sarmati Loredana,Andreoni Massimo]AIDS.2010 Jan 28;24(3):473-5. doi: 10.1097/QAD.0b013e328334cc4a. PMID: 20087078
13: Severe rhabdomyolysis associated with raltegravir use.
[Zembower Teresa R,Gerzenshtein Lana,Coleman Karen,Palella Frank J]AIDS.2008 Jul 11;22(11):1382-4. doi: 10.1097/QAD.0b013e328303be40. PMID: 18580624
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.