Search for drugs:
Typing the drug name to query
NELARABINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of ARRANON. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Infections and Infestations: Fatal opportunistic infections.
- Metabolism and Nutrition Disorders: Tumor lysis syndrome.
- Nervous System Disorders: Demyelination and ascending peripheral neuropathies similar in appearance to Guillain-Barré syndrome.
- Musculoskeletal and Connective Disorders: Rhabdomyolysis, blood creatine phosphokinase increased.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
21
42891
Other ADRs
475
14116804
Odds Ratio = 14.552
Drug Property Information
ATC Code(s):
- L01BB07 - nelarabine
- L01BB - Purine analogues
- L01B - ANTIMETABOLITES
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:nelarabine
Active Ingredient UNII:60158CV180
Drugbank ID:DB01280
PubChem Compound:3011155
CAS Number:121032-29-9
Dosage Form(s):injection
Route(s) Of Administrator:intravenous
Daily Dose:
Chemical Structure: 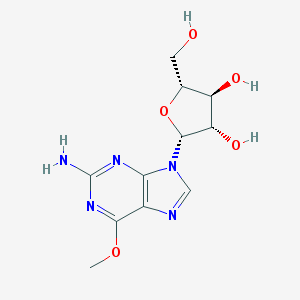

SMILE Code:
COC1=NC(=NC2=C1N=CN2[C@H]3[C@H]([C@@H]([C@H](O3)CO)O)O)N
COC1=NC(=NC2=C1N=CN2[C@H]3[C@H]([C@@H]([C@H](O3)CO)O)O)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Nelarabine associated myotoxicity and rhabdomyolysis.
[Haider Mahnur,Rizvi Syed Ahsan,Kasi Pashtoon Murtaza]Case Rep Hematol.2015;2015:825670. doi: 10.1155/2015/825670. Epub 2015 Mar 18. PMID: 25866685
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.