Search for drugs:
Typing the drug name to query
LURASIDONE HYDROCHLORIDE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- OVERDOSAGE
- Cardiovascular monitoring should commence immediately, including continuous electrocardiographic monitoring for possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide, and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with an acute overdose of LATUDA. Similarly, the alpha-blocking properties of bretylium might be additive to those of LATUDA, resulting in problematic hypotension.
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- ECG Changes
- The effects of LATUDA on the QTc interval were evaluated in a randomized, double-blind, multiple-dose, parallel-dedicated thorough QT study in 43 patients with schizophrenia or schizoaffective disorder, who were treated with LATUDA doses of 120 mg daily, 600 mg daily and completed the study. The maximum mean (upper 1-sided, 95% CI) increase in baseline-adjusted QTc intervals based on individual correction method (QTcI) was 7.5 (11.7) ms and 4.6 (9.5) ms, for the 120 mg and 600 mg dose groups respectively, observed at 2 to 4 hours after dosing. In this study, there was no apparent dose (exposure)-response relationship.
- In short-term, placebo-controlled studies in schizophrenia and bipolar depression, no post-baseline QT prolongations exceeding 500 msec were reported in patients treated with LATUDA or placebo.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
5
42907
Other ADRs
2149
14115130
Odds Ratio = 0.766
Drug Property Information
ATC Code(s):
- N05AE05 - lurasidone hydrochloride
- N05AE - Indole derivatives
- N05A - ANTIPSYCHOTICS
- N05 - PSYCHOLEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:lurasidone hydrochloride
Active Ingredient UNII:O0P4I5851I
Drugbank ID:DB08815
PubChem Compound:213046
CAS Number:367514-87-2
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 60.0 mg/day N05AE05
Chemical Structure: 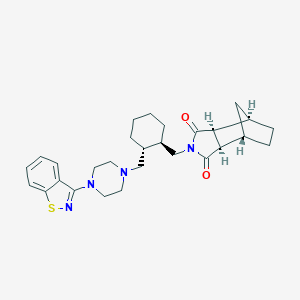

SMILE Code:
C1CC[C@H]([C@@H](C1)CN2CCN(CC2)C3=NSC4=CC=CC=C43)CN5C(=O)[C@H]6[C@@H]7CC[C@@H](C7)[C@H]6C5=O
C1CC[C@H]([C@@H](C1)CN2CCN(CC2)C3=NSC4=CC=CC=C43)CN5C(=O)[C@H]6[C@@H]7CC[C@@H](C7)[C@H]6C5=O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.