Search for drugs:
Typing the drug name to query
FENOFIBRIC ACID
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Skeletal Muscle
- Fibrates increase the risk for myopathy and have been associated with rhabdomyolysis. The risk for serious muscle toxicity appears to be increased in elderly patients and in patients with diabetes, renal failure, or hypothyroidism.
- Data from observational studies suggest that the risk for rhabdomyolysis is increased when fibrates, in particular gemfibrozil, are co-administered with an HMG-CoA reductase inhibitor (statin). The combination should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination [see CLINICAL PHARMACOLOGY (12.3)].
- Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevations of creatine phosphokinase levels.
- Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. CPK levels should be assessed in patients reporting these symptoms, and FIBRICOR therapy should be discontinued if markedly elevated CPK levels occur or myopathy/myositis is suspected or diagnosed.
- Cases of myopathy, including rhabdomyolysis, have been reported with fenofibrates co-administered with colchicine, and caution should be exercised when prescribing fenofibrate with colchicine [see DRUG INTERACTIONS (7.4)].
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during postapproval use of fenofibrate: myalgia, rhabdomyolysis, pancreatitis, muscle spasm, acute renal failure, hepatitis, cirrhosis, anemia, headache, arthralgia, decreases in hemoglobin, decreases in hematocrit, white blood cell decreases, asthenia, and severely depressed HDL-cholesterol levels. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- DRUG INTERACTIONS
- Colchicine
- Cases of myopathy, including rhabdomyolysis, have been reported with fenofibrates co-administered with colchicine, and caution should be exercised when prescribing fenofibrate with colchicine.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
44
42868
Other ADRs
2101
14115178
Odds Ratio = 6.896
Drug Property Information
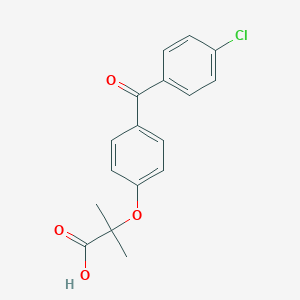
SMILE Code:
CC(C)(C(=O)O)OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl
CC(C)(C(=O)O)OC1=CC=C(C=C1)C(=O)C2=CC=C(C=C2)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study.
[Roth Eli M,McKenney James M,Kelly Maureen T,Setze Carolyn M,Carlson Dawn M,Gold Alex,Stolzenbach James C,Williams Laura A,Jones Peter H]Am J Cardiovasc Drugs.2010;10(3):175-86. doi: 10.2165/11533430-000000000-00000. PMID: 20524719
2: Fibrate therapy in the management of dyslipidemias, alone and in combination with statins: role of delayed-release fenofibric acid.
[Schima Susan M,Maciejewski Stephanie R,Hilleman Daniel E,Williams Mark A,Mohiuddin Syed M]Expert Opin Pharmacother.2010 Apr;11(5):731-8. doi: 10.1517/14656560903575639. PMID: 20210682
3: Year two assessment of fenofibric acid and moderate-dose statin combination: a phase 3, open-label, extension study.
[Kipnes Mark S,Roth Eli M,Rhyne James M,Setze Carolyn M,Lele Aditya,Kelly Maureen T,Sleep Darryl J,Stolzenbach James C]Clin Drug Investig.2010;30(1):51-61. doi: 10.2165/11319800-000000000-00000. PMID: 19995098
4: Efficacy and safety of fenofibric acid in combination with a statin in patients with mixed dyslipidemia: Pooled analysis of three phase 3, 12-week randomized, controlled studies.
[Jones Peter H,Davidson Michael H,Goldberg Anne C,Pepine Carl J,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Lele Aditya,Sleep Darryl J,Stolzenbach James C]J Clin Lipidol.2009 Apr;3(2):125-37. doi: 10.1016/j.jacl.2009.02.007. Epub 2009 Feb 11. PMID: 21291802
5: Efficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia.
[Goldberg Anne C,Bays Harold E,Ballantyne Christie M,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Am J Cardiol.2009 Feb 15;103(4):515-22. doi: 10.1016/j.amjcard.2008.10.025. Epub 2008 Dec 26. PMID: 19195513
6: Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study.
[Mohiuddin Syed M,Pepine Carl J,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Am Heart J.2009 Jan;157(1):195-203. doi: 10.1016/j.ahj.2008.08.027. Epub 2008 Nov 20. PMID: 19081418
7: Long-term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia.
[Bays Harold E,Jones Peter H,Mohiuddin Syed M,Kelly Maureen T,Sun Hsiaoming,Setze Carolyn M,Buttler Susan M,Sleep Darryl J,Stolzenbach James C]J Clin Lipidol.2008 Dec;2(6):426-35. doi: 10.1016/j.jacl.2008.10.001. Epub 2008 Nov 12. PMID: 21291776
8: Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study.
[Jones Peter H,Davidson Michael H,Kashyap Moti L,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Atherosclerosis.2009 May;204(1):208-15. doi: 10.1016/j.atherosclerosis.2008.09.027. Epub 2008 Oct 5. PMID: 18996523
9: Influence of lipid lowering fibrates on P-glycoprotein activity in vitro.
[Ehrhardt Manuela,Lindenmaier Heike,Burhenne Juergen,Haefeli Walter Emil,Weiss Johanna]Biochem Pharmacol.2004 Jan 15;67(2):285-92. PMID: 14698041
10: Micronized fenofibrate: a new fibric acid hypolipidemic agent.
[Guay D R]Ann Pharmacother.1999 Oct;33(10):1083-103. PMID: 10534222
11: [Thyroid myopathy disclosed by a fibrate].
[Schlienger J L,Goichot B,Grunenberger F,Pradignac A]Rev Med Interne.1997;18(2):169-70. PMID: 9092037
12: [Rhabdomyolysis with acute renal insufficiency: role of the association of fibrate and hypothyroidism].
[Fredenrich A,Sadoul J L,Jambou P,Vinti H]Rev Med Interne.1991 May-Jun;12(3):238. PMID: 1896719
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.