Search for drugs:
Typing the drug name to query
ROSUVASTATIN CALCIUM
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Skeletal Muscle Effects
- Cases of myopathy and rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with HMG-CoA reductase inhibitors, including rosuvastatin. These risks can occur at any dose level, but are increased at the highest dose (40 mg).
- Rosuvastatin should be prescribed with caution in patients with predisposing factors for myopathy (e.g., age ≥ 65 years, inadequately treated hypothyroidism, renal impairment).
- The risk of myopathy during treatment with rosuvastatin may be increased with concurrent administration of some other lipid-lowering therapies (fibrates or niacin), gemfibrozil, cyclosporine, atazanavir/ritonavir, lopinavir/ritonavir, or simeprevir [see DOSAGE AND ADMINISTRATION (2) and DRUG INTERACTIONS (7)]. Cases of myopathy, including rhabdomyolysis, have been reported with HMG-CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine [see DRUG INTERACTIONS (7.7)].
- Rosuvastatin therapy should be discontinued if markedly elevated creatine kinase levels occur or myopathy is diagnosed or suspected. Rosuvastatin therapy should also be temporarily withheld in any patient with an acute, serious condition suggestive of myopathy or predisposing to the development of renal failure secondary to rhabdomyolysis (e.g., sepsis, hypotension, dehydration, major surgery, trauma, severe metabolic, endocrine, and electrolyte disorders, or uncontrolled seizures).
- There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; improvement with immunosuppressive agents.
- All patients should be advised to promptly report to their physician unexplained muscle pain, tenderness, or weakness, particularly if accompanied by malaise or fever or if muscle signs and symptoms persist after discontinuing rosuvastatin.
- ADVERSE REACTIONS
- The following serious adverse reactions are discussed in greater detail in other sections of the label:
- • Rhabdomyolysis with myoglobinuria and acute renal failure and myopathy (including myositis) [see WARNINGS AND PRECAUTIONS (5.1)].
- • Liver enzyme abnormalities [see WARNINGS AND PRECAUTIONS (5.2)].
- DRUG INTERACTIONS
- Gemfibrozil
- Gemfibrozil significantly increased rosuvastatin exposure. Due to an observed increased risk of myopathy/rhabdomyolysis, combination therapy with rosuvastatin and gemfibrozil should be avoided. If used together, the dose of rosuvastatin should not exceed 10 mg once daily [see CLINICAL PHARMACOLOGY (12.3)].
- Colchicine
- Cases of myopathy, including rhabdomyolysis, have been reported with HMG‑CoA reductase inhibitors, including rosuvastatin, coadministered with colchicine, and caution should be exercised when prescribing rosuvastatin with colchicine [see WARNINGS AND PRECAUTIONS (5.1)].
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
1595
41317
Other ADRs
34183
14083096
Odds Ratio = 15.905
Drug Property Information
ATC Code(s):
- C10BX10 - rosuvastatin calcium
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BX05 - rosuvastatin calcium
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10AA07 - rosuvastatin calcium
- C10AA - HMG CoA reductase inhibitors
- C10A - "LIPID MODIFYING AGENTS, PLAIN"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BA06 - rosuvastatin calcium
- C10BA - HMG CoA reductase inhibitors in combination with other lipid modifying agents
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BX07 - rosuvastatin calcium
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C10BX09 - rosuvastatin calcium
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:rosuvastatin calcium
Active Ingredient UNII:83MVU38M7Q
Drugbank ID:DB01098
PubChem Compound:446157
CAS Number:287714-41-4
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day C10AA07
Chemical Structure: 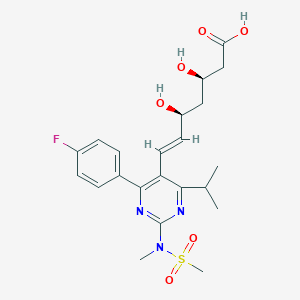

SMILE Code:
CC(C)C1=NC(=NC(=C1/C=C/[C@H](C[C@H](CC(=O)O)O)O)C2=CC=C(C=C2)F)N(C)S(=O)(=O)C
CC(C)C1=NC(=NC(=C1/C=C/[C@H](C[C@H](CC(=O)O)O)O)C2=CC=C(C=C2)F)N(C)S(=O)(=O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Pharmacogenomics and tailored polypharmacy: an 80-year-old lady with rosuvastatin-associated rhabdomyolysis and maprotiline-related Ogilvie's syndrome .
[Sreter Katherina B,Barisic Blazenka,Popovic-Grle Sanja]Int J Clin Pharmacol Ther.2017 May;55(5):442-448. doi: 10.5414/CP202784. PMID: 28257284
2: Renal Tubular Toxicity Associated With Rosuvastatin Therapy.
[Ward Frank L,John Rohan,Bargman Joanne M,McQuillan Rory F]Am J Kidney Dis.2017 Mar;69(3):473-476. doi: 10.1053/j.ajkd.2016.08.037. Epub 2016 Nov 14. PMID: 27856086
3: A phase I study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies.
[Goss Glenwood D,Jonker Derek J,Laurie Scott A,Weberpals Johanne I,Oza Amit M,Spaans Johanna N,la Porte Charles,Dimitroulakos Jim]J Transl Med.2016 Mar 31;14:83. doi: 10.1186/s12967-016-0836-6. PMID: 27036206
4: When Nutraceuticals Reinforce Drugs Side Effects: A Case Report.
[Russo Roberto,Gallelli Luca,Cannataro Roberto,Perri Mariarita,Calignano Antonio,Citraro Rita,Russo Emilio,Gareri Pietro,Corsonello Andrea,Sarro Giovambattista De]Curr Drug Saf.2016;11(3):264-6. PMID: 26830519
5: Factors Affecting the Timing of Signal Detection of Adverse Drug Reactions.
[Hashiguchi Masayuki,Imai Shungo,Uehara Keiko,Maruyama Junya,Shimizu Mikiko,Mochizuki Mayumi]PLoS One.2015 Dec 7;10(12):e0144263. doi: 10.1371/journal.pone.0144263. eCollection 2015. PMID: 26641634
6: Statin desensitization in a patient with probable familial hypercholesterolemia.
[Schultz Amy E,Snider Melissa J,Blais Danielle M,Gulati Martha]J Clin Lipidol.2015 Jul-Aug;9(4):597-601. doi: 10.1016/j.jacl.2015.03.004. Epub 2015 Mar 19. PMID: 26228679
7: Successful reintroduction of statin therapy after statin-associated rhabdomyolysis.
[Simons Janet E,Holbrook Anne M,Don-Wauchope Andrew C]J Clin Lipidol.2015 Jul-Aug;9(4):594-6. doi: 10.1016/j.jacl.2015.03.005. Epub 2015 Mar 28. PMID: 26228678
8: Rhabdomyolysis-induced acute kidney injury in a cancer patient exposed to denosumab and abiraterone: a case report.
[Neyra Javier A,Rocha Natalia A,Bhargava Rhea,Vaidya Omkar U,Hendricks Allen R,Rodan Aylin R]BMC Nephrol.2015 Jul 30;16:118. doi: 10.1186/s12882-015-0113-6. PMID: 26220655
9: Ticagrelor-induced renal failure leading to statin-induced rhabdomyolysis.
[van Vuren A J,de Jong B,Bootsma H P R,Van der Veen M J,Feith G W]Neth J Med.2015 Mar;73(3):136-8. PMID: 25852115
10: Rosuvastatin: winner in the statin wars, patients' health notwithstanding.
[Wolfe Sidney]BMJ.2015 Mar 17;350:h1388. doi: 10.1136/bmj.h1388. PMID: 25787130
11: Takotsubo cardiomyopathy with involvement of delayed-onset rhabdomyolysis and acute kidney injury after rosuvastatin treatment.
[Kamada Tomohito,Hayashi Mutsuharu,Yokoi Hiroatsu,Fujiwara Wakaya,Yoshikawa Daiji,Mukaide Daisuke,Sugishita Yoshinori,Yoshinaga Masataka,Ito Takehiro,Ozaki Yukio,Izawa Hideo]Intern Med.2015;54(1):31-5. doi: 10.2169/internalmedicine.54.3239. Epub 2015 Jan 1. PMID: 25742890
12: Risk identification and possible countermeasures for muscle adverse effects during statin therapy.
[Magni Paolo,Macchi Chiara,Morlotti Beatrice,Sirtori Cesare R,Ruscica Massimiliano]Eur J Intern Med.2015 Mar;26(2):82-8. doi: 10.1016/j.ejim.2015.01.002. Epub 2015 Jan 29. PMID: 25640999
13: Risk of adverse events among older adults following co-prescription of clarithromycin and statins not metabolized by cytochrome P450 3A4.
[Li Daniel Q,Kim Richard,McArthur Eric,Fleet Jamie L,Bailey David G,Juurlink David,Shariff Salimah Z,Gomes Tara,Mamdani Muhammad,Gandhi Sonja,Dixon Stephanie,Garg Amit X]CMAJ.2015 Feb 17;187(3):174-80. doi: 10.1503/cmaj.140950. Epub 2014 Dec 22. PMID: 25534598
14: Statins for the prevention of contrast-induced acute kidney injury.
[Ball Timothy,McCullough Peter A]Nephron Clin Pract.2014;127(1-4):165-71. doi: 10.1159/000363202. Epub 2014 Sep 24. PMID: 25343843
15: A generalised sensation of coldness following introduction of rosuvastatin therapy.
[Huynh Niem Tu,Huot Philippe]BMJ Case Rep.2014 Oct 9;2014. pii: bcr2014205987. doi: 10.1136/bcr-2014-205987. PMID: 25301422
16: Incidence of skeletal muscle disorders after statins' treatment: consequences in clinical and EMG picture.
[Drobny Michal,Pullmann Rudolf,Odalos Ivan,Skerenova Maria,Saniova Beata]Neuro Endocrinol Lett.2014;35(2):123-8. PMID: 24878976
17: Predictors and outcomes of increases in creatine phosphokinase concentrations or rhabdomyolysis risk during statin treatment.
[van Staa Tjeerd P,Carr Daniel F,O'Meara Helen,McCann Gerry,Pirmohamed Munir]Br J Clin Pharmacol.2014 Sep;78(3):649-59. doi: 10.1111/bcp.12367. PMID: 24602118
18: A timely reminder about the concomitant use of fusidic acid with statins.
[Cowan Raquel,Johnson Paul D R,Urbancic Karen,Grayson M Lindsay]Clin Infect Dis.2013 Jul;57(2):329-30. doi: 10.1093/cid/cit236. Epub 2013 Apr 15. PMID: 23588554
19: Statins and daptomycin: safety assessment of concurrent use and evaluation of drug interaction liability.
[Golightly Larry K,Barber Gerard R,Barron Michelle A,Page Robert L]Drug Metabol Drug Interact.2013;28(1):49-58. doi: 10.1515/dmdi-2012-0033. PMID: 23314530
20: Rhabdomyolysis and acute renal failure following hard physical activity in a patient treated with rosuvastatin.
[Martínez-López Diana,Enríquez Ricardo,Sirvent Ana E,Redondo-Pachón M Dolores,Millán Isabel,Amorós Francisco]Nefrologia.2012;32(1):127-8. doi: 10.3265/Nefrologia.pre2011.Oct.11118. PMID: 22294017
21: Non-lipid effects of rosuvastatin-fenofibrate combination therapy in high-risk Asian patients with mixed hyperlipidemia.
[Lee Sang-Hak,Cho Kyoung-Im,Kim Jang-Young,Ahn Young Keun,Rha Seung-Woon,Kim Yong-Jin,Choi Yun-Seok,Choi Si Wan,Jeon Dong Woon,Min Pil-Ki,Choi Dong-Ju,Baek Sang Hong,Kim Kwon Sam,Byun Young Sup,Jang Yangsoo]Atherosclerosis.2012 Mar;221(1):169-75. doi: 10.1016/j.atherosclerosis.2011.12.042. Epub 2012 Jan 5. PMID: 22269152
22: Daily and intermittent rosuvastatin 5 mg therapy in statin intolerant patients: an observational study.
[Meek Claire,Wierzbicki Anthony S,Jewkes Christina,Twomey Patrick J,Crook Martin A,Jones Alan,Viljoen Adie]Curr Med Res Opin.2012 Mar;28(3):371-8. doi: 10.1185/03007995.2012.657302. Epub 2012 Feb 7. PMID: 22256801
23: Extreme all-cause mortality in JUPITER requires reexamination of vital records.
[Serebruany Victor L]Cardiology.2011;120(2):84-8. doi: 10.1159/000330507. Epub 2011 Dec 2. PMID: 22142620
24: Rhabdomyolysis in an HIV-infected patient with impaired renal function concomitantly treated with rosuvastatin and lopinavir/ritonavir.
[de Kanter Clara T M M,Keuter Monique,van der Lee Manon J,Koopmans Peter P,Burger David M]Antivir Ther.2011;16(3):435-7. doi: 10.3851/IMP1747. PMID: 21555828
25: [Possible rosuvastatin-induced fatal rhabdomyolysis].
[Pérez Díaz I,Sánchez Argaiz M,Sánchez Gómez E]Farm Hosp.2011 Nov-Dec;35(6):340-1. doi: 10.1016/j.farma.2010.12.001. Epub 2011 May 6. PMID: 21530345
26: Severe rhabdomyolysis due to rosuvastatin in a liver transplant subject with human immunodeficiency virus and immunosuppressive therapy-related dyslipidemia.
[Moreno Ana,Fortún Jesús,Graus Javier,Rodriguez-Gandía Miguel A,Quereda Carmen,Pérez-Elías María J,Nuño Javier,Wikman Philip,Moreno Santiago,Bárcena Rafael]Liver Transpl.2011 Mar;17(3):331-3. doi: 10.1002/lt.22225. PMID: 21384516
27: Use of multiple international healthcare databases for the detection of rare drug-associated outcomes: a pharmacoepidemiological programme comparing rosuvastatin with other marketed statins.
[García Rodríguez Luis A,Herings Ron,Johansson Saga]Pharmacoepidemiol Drug Saf.2010 Dec;19(12):1218-24. doi: 10.1002/pds.2032. Epub 2010 Oct 4. PMID: 20922707
28: Rhabdomyolysis induced by rosuvastatin and sildenafil.
[Pennisi Giovanni,Vacante Marco,Russo Cristina,Malaguarnera Mariano]South Med J.2010 Oct;103(10):1052-4. doi: 10.1097/SMJ.0b013e3181f0e89c. PMID: 20818298
29: Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study.
[Roth Eli M,McKenney James M,Kelly Maureen T,Setze Carolyn M,Carlson Dawn M,Gold Alex,Stolzenbach James C,Williams Laura A,Jones Peter H]Am J Cardiovasc Drugs.2010;10(3):175-86. doi: 10.2165/11533430-000000000-00000. PMID: 20524719
30: Rosuvastatin-associated adverse effects and drug-drug interactions in the clinical setting of dyslipidemia.
[Kostapanos Michael S,Milionis Haralampos J,Elisaf Moses S]Am J Cardiovasc Drugs.2010;10(1):11-28. doi: 10.2165/13168600-000000000-00000. PMID: 20104931
31: Year two assessment of fenofibric acid and moderate-dose statin combination: a phase 3, open-label, extension study.
[Kipnes Mark S,Roth Eli M,Rhyne James M,Setze Carolyn M,Lele Aditya,Kelly Maureen T,Sleep Darryl J,Stolzenbach James C]Clin Drug Investig.2010;30(1):51-61. doi: 10.2165/11319800-000000000-00000. PMID: 19995098
32: Human skeletal muscle drug transporters determine local exposure and toxicity of statins.
[Knauer Michael J,Urquhart Bradley L,Meyer zu Schwabedissen Henriette E,Schwarz Ute I,Lemke Christopher J,Leake Brenda F,Kim Richard B,Tirona Rommel G]Circ Res.2010 Feb 5;106(2):297-306. doi: 10.1161/CIRCRESAHA.109.203596. Epub 2009 Nov 25. PMID: 19940267
33: Rosuvastatin-induced rhabdomyolysis probably via CYP2C9 saturation.
[Gallelli L,Ferraro M,Spagnuolo V,Rende P,Mauro G F,De Sarro G]Drug Metabol Drug Interact.2009;24(1):83-7. PMID: 19354002
34: [Statin therapy and muscle disorders].
[Abel Tatjána,Fehér János]Orv Hetil.2009 Feb 8;150(6):261-3. doi: 10.1556/OH.2009.28520. PMID: 19179258
35: Should high creatine kinase discourage the initiation or continuance of statins for the treatment of hypercholesterolemia?
[Glueck Charles J,Rawal Bishal,Khan Naseer Ahmed,Yeramaneni Samrat,Goldenberg Naila,Wang Ping]Metabolism.2009 Feb;58(2):233-8. doi: 10.1016/j.metabol.2008.09.019. PMID: 19154957
36: Rosuvastatin induced rhabdomyolysis in a low risk patient: a case report and review of the literature.
[Khan Fahmi Yousef,Ibrahim Wael]Curr Clin Pharmacol.2009 Jan;4(1):1-3. PMID: 19149497
37: A case of asymptomatic cytoplasmic body myopathy revealed by sinvastatin.
[Evangelista Teresinha,Ferro José,Pereira Pedro,de Carvalho Mamede]Neuromuscul Disord.2009 Jan;19(1):66-8. doi: 10.1016/j.nmd.2008.10.008. Epub 2008 Dec 11. PMID: 19084404
38: Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study.
[Jones Peter H,Davidson Michael H,Kashyap Moti L,Kelly Maureen T,Buttler Susan M,Setze Carolyn M,Sleep Darryl J,Stolzenbach James C]Atherosclerosis.2009 May;204(1):208-15. doi: 10.1016/j.atherosclerosis.2008.09.027. Epub 2008 Oct 5. PMID: 18996523
39: The safety of rosuvastatin in comparison with other statins in over 100,000 statin users in UK primary care.
[García-Rodríguez Luis Alberto,Massó-González Elvira Luján,Wallander Mari-Ann,Johansson Saga]Pharmacoepidemiol Drug Saf.2008 Oct;17(10):943-52. doi: 10.1002/pds.1603. PMID: 18425988
40: The safety of rosuvastatin in comparison with other statins in over 25,000 statin users in the Saskatchewan Health Databases.
[García-Rodríguez Luis Alberto,González-Pérez Antonio,Stang Mary Rose,Wallander Mari-Ann,Johansson Saga]Pharmacoepidemiol Drug Saf.2008 Oct;17(10):953-61. doi: 10.1002/pds.1602. PMID: 18425987
41: McArdle disease with rhabdomyolysis induced by rosuvastatin: case report.
[Lorenzoni Paulo José,Silvado Carlos Eduardo,Scola Rosana Herminia,Luvizotto Mario,Werneck Lineu César]Arq Neuropsiquiatr.2007 Sep;65(3B):834-7. PMID: 17952291
42: Myopathy caused by a combination rosuvastatin and fenofibrate.
[Dedhia V,Munsi S C]J Assoc Physicians India.2007 Feb;55:152-3. PMID: 17571748
43: Preventive effects of bicarbonate on cerivastatin-induced apoptosis.
[Kobayashi Masaki,Kaido Fumie,Kagawa Toshiki,Itagaki Shirou,Hirano Takeshi,Iseki Ken]Int J Pharm.2007 Aug 16;341(1-2):181-8. Epub 2007 Apr 24. PMID: 17553641
44: By the way, doctor. I am 80 and am taking a 40-milligram Crestor pill every day. Recently I saw a Crestor ad that said blood tests should be done to monitor for possible side effects of liver or muscle injury. Can you tell me something about those tests?
[Komaroff Anthony L]Harv Health Lett.2007 Mar;32(5):8. PMID: 17390496
45: Safety of rosuvastatin: update on 16,876 rosuvastatin-treated patients in a multinational clinical trial program.
[Shepherd James,Vidt Donald G,Miller Elinor,Harris Susan,Blasetto James]Cardiology.2007;107(4):433-43. Epub 2007 Mar 16. PMID: 17363845
46: Safety profile of rosuvastatin: results of a prescription-event monitoring study of 11,680 patients.
[Kasliwal Rachna,Wilton Lynda V,Cornelius Victoria,Aurich-Barrera Beate,Shakir Saad A W]Drug Saf.2007;30(2):157-70. PMID: 17253880
47: Effect of rosuvastatin 5-20mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia.
[Saito Yasushi,Yamada Nobuhiro,Shirai Kohji,Sasaki Jun,Ebihara Yoshinori,Yanase Toshihiko,Fox Jonathan C]Atherosclerosis.2007 Oct;194(2):505-11. Epub 2007 Jan 16. PMID: 17223112
48: Atorvastatin for stroke prevention.
Med Lett Drugs Ther.2006 Sep 11;48(1243):75-6. PMID: 16977287
49: Comparison of rosuvastatin versus atorvastatin in Hispanic-Americans with hypercholesterolemia (from the STARSHIP trial).
[Lloret Ramon,Ycas Joseph,Stein Michael,Haffner Steven,STARSHIP Study Group]Am J Cardiol.2006 Sep 15;98(6):768-73. Epub 2006 Jul 28. PMID: 16950182
50: Rosuvastatin: an independent analysis of risks and benefits.
[Zipes Douglas P,Zvaifler Nathan J,Glassock Richard J,Gilman Sid,Muñoz Alvaro,Gogolak Victor,Gordis Leon,Dedon Peter C,Guengerich Frederick P,Wasserman Stephen I,Witztum Joseph L,Wogan Gerald N]MedGenMed.2006 Jun 14;8(2):73. PMID: 16926812
51: Rhabdomyolysis associated with pomegranate juice consumption.
[Sorokin Alexey V,Duncan Brett,Panetta Randolph,Thompson Paul D]Am J Cardiol.2006 Sep 1;98(5):705-6. Epub 2006 Jul 14. PMID: 16923466
52: The comparative safety of rosuvastatin: a retrospective matched cohort study in over 48,000 initiators of statin therapy.
[McAfee Andrew T,Ming Eileen E,Seeger John D,Quinn Sherry G,Ng Eva W,Danielson Jared D,Cutone Jennifer A,Fox Jonathan C,Walker Alexander M]Pharmacoepidemiol Drug Saf.2006 Jul;15(7):444-53. PMID: 16761308
53: Results from a rosuvastatin historical cohort study in more than 45,000 Dutch statin users, a PHARMO study.
[Goettsch W G,Heintjes E M,Kastelein J J P,Rabelink T J,Johansson Saga,Herings R M C]Pharmacoepidemiol Drug Saf.2006 Jul;15(7):435-43. PMID: 16761304
54: Rosuvastatin: a risk-benefit assessment for intensive lipid lowering.
[Ferdinand Keith C]Expert Opin Pharmacother.2005 Sep;6(11):1897-910. PMID: 16144509
55: New warning for statin drug.
FDA Consum.2005 May-Jun;39(3):2. PMID: 16127807
56: Pharmacologic options for aggressive low-density lipoprotein cholesterol lowering: benefits versus risks.
[McKenney James M]Am J Cardiol.2005 Aug 22;96(4A):60E-66E. PMID: 16098846
57: Rhabdomyolysis with cardiac involvement and acute renal failure in a patient taking rosuvastatin and fenofibrate.
[Ireland James H E,Eggert Christoph H,Arendt Christopher J,Williams Amy W]Ann Intern Med.2005 Jun 7;142(11):949-50. PMID: 15941707
58: The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis.
[Alsheikh-Ali Alawi A,Ambrose Marietta S,Kuvin Jeffrey T,Karas Richard H]Circulation.2005 Jun 14;111(23):3051-7. Epub 2005 May 23. PMID: 15911706
59: Rosuvastatin and the statin wars--the way to peace.
[Meyboom Ronald H B,Edwards I Ralph]Lancet.2004 Dec 4-10;364(9450):1997-9. PMID: 15582043
60: Rosuvastatin: new preparation. Opt for statins with evidence of efficacy on clinical outcome.
Prescrire Int.2004 Aug;13(72):132-4. PMID: 15532136
61: Rosuvastatin safety: lessons from the FDA review and post-approval surveillance.
[Davidson Michael H]Expert Opin Drug Saf.2004 Nov;3(6):547-57. PMID: 15500414
62: Safety of rosuvastatin.
[Shepherd James,Hunninghake Donald B,Stein Evan A,Kastelein John J P,Harris Susan,Pears John,Hutchinson Howard G]Am J Cardiol.2004 Oct 1;94(7):882-8. PMID: 15464670
63: Rosuvastatin (Crestor) and rhabdomyolysis.
[Wooltorton Eric]CMAJ.2004 Jul 20;171(2):129. PMID: 15262879
64: Safety and efficacy of rosuvastatin.
[Olsson Gunnar O]Lancet.2004 Jul 10-16;364(9429):135. PMID: 15246717
65: Dangers of rosuvastatin identified before and after FDA approval.
[Wolfe Sidney M]Lancet.2004 Jun 26;363(9427):2189-90. PMID: 15220045
66: Diagnosing familial combined hyperlipidemia.
[SoRelle Ruth]Circulation.2004 Jun 22;109(24):e9052-3. PMID: 15210617
67: Pressure group urges rosuvastatin recall.
[Ault Alicia]Lancet.2004 Mar 13;363(9412):871. PMID: 15032242
68: Rosuvastatin-warfarin drug interaction.
[Barry Michael]Lancet.2004 Jan 24;363(9405):328. PMID: 14751711
69: Benefit-risk assessment of Rosuvastatin 10 to 40 milligrams.
[Brewer H Bryan]Am J Cardiol.2003 Aug 21;92(4B):23K-29K. PMID: 12948873
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.