Search for drugs:
Typing the drug name to query
KETOCONAZOLE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- BOXED WARNING
- QT Prolongation and Drug Interactions Leading to QT Prolongation
- Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, ranolazine. Ketoconazole can cause elevated plasma concentrations of these drugs and may prolong QT intervals, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes. See CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS: DRUG INTERACTIONS sections.
- WARNINGS
- QT Prolongation and Drug Interactions Leading to QT Prolongation
- Ketoconazole can prolong the QT interval. Co-administration of the following drugs with ketoconazole is contraindicated: dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, ranolazine. Ketoconazole can cause elevated plasma concentrations of these drugs which may prolong the QT interval, sometimes resulting in life-threatening ventricular dysrhythmias such as torsades de pointes.
- PRECAUTIONS
- Drug Interactions
- Drugs that may have their plasma concentrations increased by ketoconazole
- Ketoconazole can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with ketoconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with ketoconazole tablets, since the combination may lead to ventricular tachyarrhythmias, including occurrences of torsade de pointes, a potentially fatal arrhythmia.
- Drugs that may have their plasma concentrations increased by ketoconazole
- Ketoconazole can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with ketoconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with ketoconazole tablets, since the combination may lead to ventricular tachyarrhythmias, including occurrences of torsade de pointes, a potentially fatal arrhythmia.
- >>7e0bf510-af56-446b-8f31-ec61fe057392.jpeg
- CONTRAINDICATIONS
- Drug Interactions
- Coadministration of a number of CYP3A4 substrates such as dofetilide, quinidine cisapride and pimozide is contraindicated with ketoconazole tablets. Coadministration with ketoconazole can cause elevated plasma concentrations of these drugs and may increase or prolong both therapeutic and adverse effects to such an extent that a potentially serious adverse reaction may occur. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and sometimes resulting in life-threatening ventricular tachyarrhythmias including occurrences of torsades de pointes, a potentially fatal arrhythmia. (See PRECAUTIONS: DRUG INTERACTIONS.)
- Additionally, the following other drugs are contraindicated with ketoconazole tablets: methadone, disopyramide, dronedarone, ergot alkaloids such as dihydroergotamine, ergometrine, ergotamine, methylergometrine, irinotecan, lurasidone, oral midazolam, alprazolam, triazolam, felodipine, nisoldipine, ranolazine, tolvaptan, eplerenone, lovastatin, simvastatin and colchicine. (See PRECAUTIONS: DRUG INTERACTIONS.)
- CLINICAL PHARMACOLOGY
- Electrocardiogram
- Pre-clinical electrophysiological studies have shown that ketoconazole inhibits the rapidly activating component of the cardiac delayed rectifier potassium current, prolongs the action potential duration, and may prolong the QT c interval. Data from some clinical PK/PD studies and drug interaction studies suggest that oral dosing with ketoconazole at 200 mg twice daily for 3 to 7 days can result in an increase of the QT c interval: a mean maximum increase of about 6 to 12 msec was seen at ketoconazole peak plasma concentrations about 1 to 4 hours after ketoconazole administration.
- MEDICATION GUIDE
- changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heart beats that can be life threatening. This can happen when ketoconazole tablets are taken with certain medicines, such as dofetilide, quinidine, pimozide, cisapride, methadone, disopyramide, dronedarone, and ranolazine. Talk to your healthcare provider about other medicines you are taking before you start taking ketoconazole tablets. Tell your healthcare provider right away if you feel faint, lightheaded, dizzy, or feel your heart beating irregularly or fast. These may be symptoms related to QT prolongation.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
52
42860
Other ADRs
3832
14113447
Odds Ratio = 4.469
Drug Property Information
ATC Code(s):
- G01AF11 - ketoconazole
- G01AF - Imidazole derivatives
- G01A - "ANTIINFECTIVES AND ANTISEPTICS, EXCL. COMBINATIONS "
- G01 - GYNECOLOGICAL ANTIINFECTIVES AND ANTISEPTICS
- G - GENITO URINARY SYSTEM AND SEX HORMONES
- D01AC08 - ketoconazole
- D01AC - Imidazole and triazole derivatives
- D01A - ANTIFUNGALS FOR TOPICAL USE
- D01 - ANTIFUNGALS FOR DERMATOLOGICAL USE
- D - DERMATOLOGICALS
- J02AB02 - ketoconazole
- J02AB - Imidazole derivatives
- J02A - ANTIMYCOTICS FOR SYSTEMIC USE
- J02 - ANTIMYCOTICS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:ketoconazole
Active Ingredient UNII:R9400W927I
Drugbank ID:DB01026
PubChem Compound:3823
CAS Number:65277-42-1
Dosage Form(s):gel
Route(s) Of Administrator:topical
Daily Dose:
- 400.0 mg/day G01AF11
- 200.0 mg/day J02AB02
Chemical Structure: 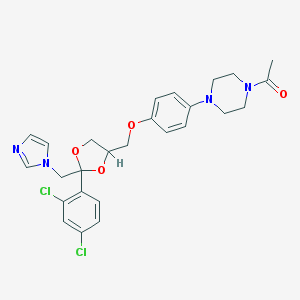

SMILE Code:
CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl
CC(=O)N1CCN(CC1)C2=CC=C(C=C2)OCC3COC(O3)(CN4C=CN=C4)C5=C(C=C(C=C5)Cl)Cl
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Weakness and pain in arms and legs · dark urine · history of vertebral osteomyelitis · Dx?
[Charokopos Antonios,Muhammad Tariq,Surbhi Sidana,Brateanu Andrei]J Fam Pract.2017 Mar;66(3):170-173. PMID: 28249055
2: Rhabdomyolysis in a Prostate Cancer Patient Taking Ketoconazole and Simvastatin: Case Report and Review of the Literature.
[Watkins Jack L,Atkinson Bradley J,Pagliaro Lance C]Ann Pharmacother.2011 Feb;45(2):e9. doi: 10.1345/aph.1P433. PMID: 21304039
3: Risk management of simvastatin or atorvastatin interactions with CYP3A4 inhibitors.
[Molden Espen,Skovlund Eva,Braathen Pia]Drug Saf.2008;31(7):587-96. PMID: 18558792
4: Tailored hormonal therapy in secretory adrenocortical cancer.
[Carmona-Bayonas A,Soler I O,Gómez F I,Billalabeitia E G,Saura H P,Tafalla M S A,Díaz M P]Ann Oncol.2007 Jul;18(7):1281. PMID: 17675396
5: A lesson for everyone in drug-drug interactions.
[Akram Kamran,Rao Swapna,Parker Miriam]Int J Cardiol.2007 May 16;118(1):e19-20. Epub 2007 Mar 21. PMID: 17368833
6: Hepatitis and rhabdomyolysis in a patient with hormone refractory prostate cancer on ketoconazole and concurrent lovastatin therapy.
[Stein C A,Goel Sanjay,Ghavamian Reza]Invest New Drugs.2007 Jun;25(3):277-8. Epub 2007 Jan 11. PMID: 17216557
7: Rhabdomyolysis from cytochrome p-450 interaction of ketoconazole and simvastatin in prostate cancer.
[Itakura Haruka,Vaughn David,Haller Daniel G,O'Dwyer Peter J]J Urol.2003 Feb;169(2):613. PMID: 12544321
8: Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors.
[Williams David,Feely John]Clin Pharmacokinet.2002;41(5):343-70. PMID: 12036392
9: [Safety profile of statins].
[Prieto J C]Rev Med Chil.2001 Nov;129(11):1237-40. PMID: 11836874
10: Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition.
[Dresser G K,Spence J D,Bailey D G]Clin Pharmacokinet.2000 Jan;38(1):41-57. PMID: 10668858
11: Rhabdomyolysis induced by simvastatin and ketoconazole treatment.
[Gilad R,Lampl Y]Clin Neuropharmacol.1999 Sep-Oct;22(5):295-7. PMID: 10516882
12: High-dose itraconazole in the treatment of severe mycoses.
[Sharkey P K,Rinaldi M G,Dunn J F,Hardin T C,Fetchick R J,Graybill J R]Antimicrob Agents Chemother.1991 Apr;35(4):707-13. PMID: 1648887
13: Fatal rhabdomyolysis as a complication of bone marrow transplantation.
[Volin L,Järventie G,Ruutu T]Bone Marrow Transplant.1990 Jul;6(1):59-60. PMID: 2390634
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.