Search for drugs:
Typing the drug name to query
ESCITALOPRAM OXALATE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- DRUG INTERACTIONS
- Pimozide and Celexa
- In a controlled study, a single dose of pimozide 2 mg co-administered with racemic citalopram 40 mg given once daily for 11 days was associated with a mean increase in QTc values of approximately 10 msec compared to pimozide given alone. Racemic citalopram did not alter the mean AUC or Cmax of pimozide. The mechanism of this pharmacodynamic interaction is not known.
- OVERDOSAGE
- Human Experience
- Symptoms most often accompanying escitalopram overdose, alone or in combination with other drugs and/or alcohol, included convulsions, coma, dizziness, hypotension, insomnia, nausea, vomiting, sinus tachycardia, somnolence, and ECG changes (including QT prolongation and very rare cases of torsade de pointes). Acute renal failure has been very rarely reported accompanying overdose.
- ADVERSE REACTIONS
- ECG Changes
- Electrocardiograms from escitalopram oxalate (N=625) and placebo (N=527) groups were compared with respect to outliers defined as subjects with QTc changes over 60 msec from baseline or absolute values over 500 msec post-dose, and subjects with heart rate increases to over 100 bpm or decreases to less than 50 bpm with a 25% change from baseline (tachycardic or bradycardic outliers, respectively). None of the patients in the escitalopram oxalate group had a QTcF interval >500 msec or a prolongation >60 msec compared to 0.2% of patients in the placebo group. The incidence of tachycardic outliers was 0.2% in the escitalopram oxalate and the placebo group. The incidence of bradycardic outliers was 0.5% in the escitalopram oxalate group and 0.2% in the placebo group.
- QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg) controlled cross-over, escalating multiple-dose study in 113 healthy subjects. The maximum mean (95% upper confidence bound) difference from placebo arm were 4.5 (6.4) and 10.7 (12.7) msec for 10 mg and supratherapeutic 30 mg escitalopram given once daily, respectively. Based on the established exposure-response relationship, the predicted QTcF change from placebo arm (95% confidence interval) under the Cmax for the dose of 20 mg is 6.6 (7.9) msec. Escitalopram 30 mg given once daily resulted in mean Cmax of 1.7-fold higher than the mean Cmax for the maximum recommended therapeutic dose at steady state (20 mg). The exposure under supratherapeutic 30 mg dose is similar to the steady state concentrations expected in CYP2C19 poor metabolizers following a therapeutic dose of 20 mg.
- Investigations: bilirubin increased, decreased weight, electrocardiogram QT prolongation, hepatic enzymes increased, hypercholesterolemia, INR increased, prothrombin decreased.
- NONCLINICAL TOXICOLOGY
- Cardiovascular Changes in Dogs
- In a one-year toxicology study, 5 of 10 beagle dogs receiving oral racemic citalopram doses of 8 mg/kg/day died suddenly between weeks 17 and 31 following initiation of treatment. Sudden deaths were not observed in rats at doses of racemic citalopram up to 120 mg/kg/day, which produced plasma levels of citalopram and its metabolites demethylcitalopram and didemethylcitalopram (DDCT) similar to those observed in dogs at 8 mg/kg/day. A subsequent intravenous dosing study demonstrated that in beagle dogs, racemic DDCT caused QT prolongation, a known risk factor for the observed outcome in dogs.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
81
42831
Other ADRs
13687
14103592
Odds Ratio = 1.949
Drug Property Information
ATC Code(s):
- N06AB10 - escitalopram oxalate
- N06AB - Selective serotonin reuptake inhibitors
- N06A - ANTIDEPRESSANTS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:escitalopram oxalate
Active Ingredient UNII:5U85DBW7LO
Drugbank ID:DB01175
PubChem Compound:146570
CAS Number:128196-01-0
Dosage Form(s):solution
Route(s) Of Administrator:oral
Daily Dose:
- 10.0 mg/day N06AB10
Chemical Structure: 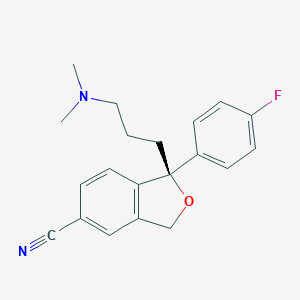

SMILE Code:
CN(C)CCC[C@@]1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F
CN(C)CCC[C@@]1(C2=C(CO1)C=C(C=C2)C#N)C3=CC=C(C=C3)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.