Search for drugs:
Typing the drug name to query
SIROLIMUS
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Hyperlipidemia
- Increased serum cholesterol and triglycerides requiring treatment occurred more frequently in patients treated with sirolimus compared with azathioprine or placebo controls in Studies 1 and 2 [see ADVERSE REACTIONS (6.1)]. There were increased incidences of hypercholesterolemia (43 to 46%) and/or hypertriglyceridemia (45 to 57%) in patients receiving sirolimus compared with placebo controls (each 23%). The risk/benefit should be carefully considered in patients with established hyperlipidemia before initiating an immunosuppressive regimen including sirolimus.
- Any patient who is administered sirolimus should be monitored for hyperlipidemia. If detected, interventions such as diet, exercise, and lipid-lowering agents should be initiated as outlined by the National Cholesterol Education Program guidelines.
- In clinical trials of patients receiving sirolimus plus cyclosporine or sirolimus after cyclosporine withdrawal, up to 90% of patients required treatment for hyperlipidemia and hypercholesterolemia with anti-lipid therapy (e.g., statins, fibrates). Despite anti-lipid management, up to 50% of patients had fasting serum cholesterol levels > 240 mg/dL and triglycerides above recommended target levels. The concomitant administration of sirolimus and HMG-CoA reductase inhibitors resulted in adverse reactions such as CPK elevations (3%), myalgia (6.7%) and rhabdomyolysis (< 1%). In these trials, the number of patients was too small and duration of follow-up too short to evaluate the long-term impact of sirolimus on cardiovascular mortality.
- During sirolimus therapy with or without cyclosporine, patients should be monitored for elevated lipids, and patients administered an HMG-CoA reductase inhibitor and/or fibrate should be monitored for the possible development of rhabdomyolysis and other adverse effects, as described in the respective labeling for these agents.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
36
42876
Other ADRs
9322
14107957
Odds Ratio = 1.271
Drug Property Information
ATC Code(s):
- S01XA23 - sirolimus
- S01XA - Other ophthalmologicals
- S01X - OTHER OPHTHALMOLOGICALS
- S01 - OPHTHALMOLOGICALS
- S - SENSORY ORGANS
- L04AA10 - sirolimus
- L04AA - Selective immunosuppressants
- L04A - IMMUNOSUPPRESSANTS
- L04 - IMMUNOSUPPRESSANTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:sirolimus
Active Ingredient UNII:W36ZG6FT64
Drugbank ID:DB00877
PubChem Compound:5284616
CAS Number:53123-88-9
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 3.0 mg/day L04AA10
Chemical Structure: 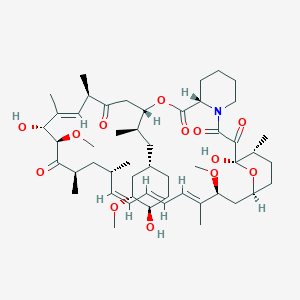

SMILE Code:
C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)O)C)/C)O)OC)C)C)/C)OC
C[C@@H]1CC[C@H]2C[C@@H](/C(=C/C=C/C=C/[C@H](C[C@H](C(=O)[C@@H]([C@@H](/C(=C/[C@H](C(=O)C[C@H](OC(=O)[C@@H]3CCCCN3C(=O)C(=O)[C@@]1(O2)O)[C@H](C)C[C@@H]4CC[C@H]([C@@H](C4)OC)O)C)/C)O)OC)C)C)/C)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis as a clinical manifestation of association with ciprofibrate, sirolimus, cyclosporine, and pegylated interferon-α in liver-transplanted patients: a case report and literature review.
[dos Santos A G,Guardia A C,Pereira T S,Ataíde E C,Mei M d F T,Udo M E,Boin I F S F,Stucchi R S B]Transplant Proc.2014 Jul-Aug;46(6):1887-8. doi: 10.1016/j.transproceed.2014.05.065. PMID: 25131061
2: Severe rhabdomyolysis associated with concurrent use of simvastatin and sirolimus after cisplatin-based chemotherapy in a kidney transplant recipient.
[Hong Yu Ah,Kim Hyung Duk,Jo Kwanhoon,Park Yun Kyung,Lee Jonghoon,Sun In O,Chung Byung Ha,Park Cheol Whee,Yang Chul Woo,Choi Bum Soon]Exp Clin Transplant.2014 Apr;12(2):152-5. doi: 10.6002/ect.2013.0003. Epub 2013 May 29. PMID: 23734754
3: Ezetimibe is effective in the treatment of persistent hyperlipidemia of renal allograft recipients.
[Savvidaki E,Koukoulaki M,Benou A,Roumeliotou M,Fourtounas C,Kalliakmani P,Papachristou E,Vlachojannis J G,Goumenos D]Clin Nephrol.2011 Feb;75(2):107-12. PMID: 21255539
4: Regression of subependymal giant cell astrocytomas with RAD001 (Everolimus) in tuberous sclerosis complex.
[Yalon Michal,Ben-Sira L,Constantini S,Toren A]Childs Nerv Syst.2011 Jan;27(1):179-81. doi: 10.1007/s00381-010-1222-y. Epub 2010 Aug 12. PMID: 20703486
5: Rhabdomyolysis and acute kidney injury secondary to concomitant use of fluvastatin and rapamycin in a renal transplant recipient.
[Basic-Jukic Nikolina,Kes Petar,Bubic-Filipi Ljubica,Vranjican Zoran]Nephrol Dial Transplant.2010 Jun;25(6):2036; author reply 2036-7. doi: 10.1093/ndt/gfq157. Epub 2010 Mar 23. PMID: 20332417
6: Severe rhabdomyolysis and acute renal failure in a kidney transplant patient treated with tacrolimus and chimaeric CD25 monoclonal antibody.
[Fontana I,Ginevri F,Basile G,Beatini M,Bertocchi M,Bonifazio L,Saltalamacchia L,Ghinolfi D,Santori G,Valente R,Perfumo F,Valente U]Transplant Proc.2004 Apr;36(3):711-2. PMID: 15110640
7: Drug-related dyslipidemia after renal transplantation.
[Mathis A Scott,Davé Nisha,Knipp Gregory T,Friedman Gary S]Am J Health Syst Pharm.2004 Mar 15;61(6):565-85; quiz 586-7. PMID: 15061429
8: [Metabolic modifications related to immunosuppressive drugs].
[Ducobu J]Rev Med Brux.2002 Jun;23(3):156-9. PMID: 12143154
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.