Search for drugs:
Typing the drug name to query
DESFLURANE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-Marketing Experience
- The following adverse reactions have been identified during post-approval use of SUPRANE. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: Coagulopathy
- Metabolism and Nutrition Disorders: Hyperkalemia, Hypokalemia, metabolic acidosis
- Nervous System Disorders: Convulsion, Post-operative agitation in children
- Eye Disorders: Ocular icterus
- Cardiac Disorders: Cardiac arrest, Torsade de pointes, ventricular failure, ventricular hypokinesia, atrial fibrillation
- Vascular Disorders: Malignant hypertension, hemorrhage, hypotension, shock
- Respiratory, Thoracic and Mediastinal Disorders: Respiratory arrest, respiratory failure, respiratory distress, bronchospasm, hemoptysis
- Gastrointestinal Disorders: Pancreatitis acute, abdominal pain
- Hepatobiliary Disorders: Hepatic failure, hepatic necrosis, hepatitis, cytolytic hepatitis, cholestasis, jaundice, hepatic function abnormal, liver disorder
- Skin and Subcutaneous Tissue Disorder: Urticaria, erythema
- Musculoskeletal, Connective Tissue and Bone Disorders: Rhabdomyolysis
- General Disorders and Administration Site Conditions: Hyperthermia malignant, asthenia, malaise
- Investigations: Electrocardiogram ST-T change, electrocardiogram T-wave inversion, tranaminases increased, alanine aminotransferase increased, aspartate aminotransferase increased, blood bilirubin increased, coagulation test abnormal, ammonia increased
- Injury, Poisoning, and Procedural Complications*: Tachyarrhythmia, palpitations, eye burns, blindness transient, encephalopathy, ulcerative keratitis, ocular hyperemia, visual acuity reduced, eye irritation, eye pain, dizziness, migraine, fatigue, accidental exposure, skin burning sensation, drug administration error
- *Reactions categorized within this SOC were accidental exposures to non-patients.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
5
42907
Other ADRs
640
14116639
Odds Ratio = 2.571
Drug Property Information
ATC Code(s):
- N01AB07 - desflurane
- N01AB - Halogenated hydrocarbons
- N01A - "ANESTHETICS, GENERAL"
- N01 - ANESTHETICS
- N - NERVOUS SYSTEM
Active Ingredient:desflurane
Active Ingredient UNII:CRS35BZ94Q
Drugbank ID:DB01189
PubChem Compound:42113
CAS Number:57041-67-5
Dosage Form(s):liquid
Route(s) Of Administrator:respiratory (inhalation)
Daily Dose:
Chemical Structure: 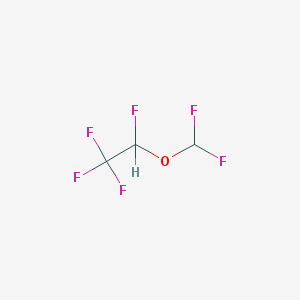

SMILE Code:
C(C(F)(F)F)(OC(F)F)F
C(C(F)(F)F)(OC(F)F)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Malignant hyperthermia: a review.
[Rosenberg Henry,Pollock Neil,Schiemann Anja,Bulger Terasa,Stowell Kathryn]Orphanet J Rare Dis.2015 Aug 4;10:93. doi: 10.1186/s13023-015-0310-1. PMID: 26238698
2: Delayed onset malignant hyperthermia after sevoflurane.
[Turhan K Sanem Cakar,Baytaş Volkan,Batislam Yeşim,Ozatamer Oya]Case Rep Anesthesiol.2013;2013:712710. doi: 10.1155/2013/712710. Epub 2013 May 30. PMID: 23819066
3: Malignant hyperthermia.
[Rosenberg Henry,Davis Mark,James Danielle,Pollock Neil,Stowell Kathryn]Orphanet J Rare Dis.2007 Apr 24;2:21. PMID: 17456235
4: Anesthetic effects on the glycerol model of rhabdomyolysis-induced acute renal failure in rats.
[Lochhead K M,Kharasch E D,Zager R A]J Am Soc Nephrol.1998 Feb;9(2):305-9. PMID: 9527408
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.