Search for drugs:
Typing the drug name to query
DEUTETRABENAZINE
DIR Classification
Classification:Most-DIR concern
Severity Score:4
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- QTc Prolongation
- AUSTEDO may prolong the QT interval, but the degree of QT prolongation is not clinically significant when AUSTEDO is administered within the recommended dosage range [see Clinical Pharmacology (12.2)].
- AUSTEDO should be avoided in patients with congenital long QT syndrome and in patients with a history of cardiac arrhythmias. Certain circumstances may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
- ADVERSE REACTIONS
- The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Depression and Suicidality in Patients with Huntington’s disease [see Warnings and Precautions (5.1)]
- QTc Prolongation [see Warnings and Precautions (5.3)]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions (5.4)]
- Akathisia, Agitation, and Restlessness [see Warnings and Precautions (5.5)]
- Parkinsonism [see Warnings and Precautions (5.6)]
- Sedation and Somnolence [see Warnings and Precautions (5.7)]
- Hyperprolactinemia [see Warnings and Precautions (5.8)]
- Binding to Melanin-Containing Tissues [see Warnings and Precautions (5.9)]
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At the maximum recommended dose, AUSTEDO does not prolong the QT interval to any clinically relevant extent. An exposure-response analysis on QTc prolongation from a study in extensive or intermediate (EM) and poor CYP2D6 metabolizers (PM) showed that a clinically-relevant effect can be excluded at exposures following single doses of 24 and 48 mg of AUSTEDO.
- PATIENT COUNSELING INFORMATION
- Prolongation of the QTc Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations [see Warnings and Precautions (5.3)]. Advise patients to inform physicians that they are taking AUSTEDO before any new drug is taken.
- MEDICATION GUIDE
- Before taking AUSTEDO, tell your healthcare provider about all of your medical conditions, including if you:
- have emotional or mental problems (for example, depression, nervousness, anxiety, anger, agitation, psychosis, previous suicidal thoughts or suicide attempts).
- have liver disease.
- have an irregular heart rhythm or heartbeat (QT prolongation, cardiac arrhythmia) or a heart problem called congenital long QT syndrome.
- have low levels of potassium or magnesium in your blood (hypokalemia or hypomagnesemia).
- have breast cancer or a history of breast cancer.
- are pregnant or plan to become pregnant. It is not known if AUSTEDO can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AUSTEDO passes into breast milk.
- AUSTEDO can cause serious side effects, including:
- Irregular heartbeat (QT prolongation). AUSTEDO increases your chance of having certain changes in the electrical activity in your heart. These changes can lead to a dangerous abnormal heartbeat. Taking AUSTEDO with certain medicines may increase this chance.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
4
14117275
Odds Ratio = N/A
Drug Property Information
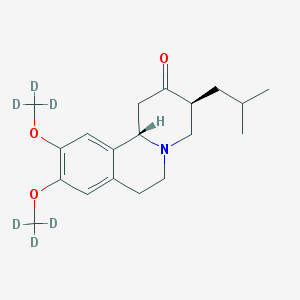
SMILE Code:
[2H]C([2H])([2H])OC1=C(C=C2[C@@H]3CC(=O)[C@H](CN3CCC2=C1)CC(C)C)OC([2H])([2H])[2H]
[2H]C([2H])([2H])OC1=C(C=C2[C@@H]3CC(=O)[C@H](CN3CCC2=C1)CC(C)C)OC([2H])([2H])[2H]
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.