Search for drugs:
Typing the drug name to query
SONIDEGIB
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Cardiac Electrophysiology
- At a dose of 800 mg once daily, sonidegib does not prolong the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
0
42912
Other ADRs
42
14117237
Odds Ratio = N/A
Drug Property Information
ATC Code(s):
- L01XX48 - sonidegib
- L01XX - Other antineoplastic agents
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:sonidegib phosphate
Active Ingredient UNII:W421AI34UW
Drugbank ID:DB09143
PubChem Compound:24775005
CAS Number:956697-53-3
Dosage Form(s):capsule
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 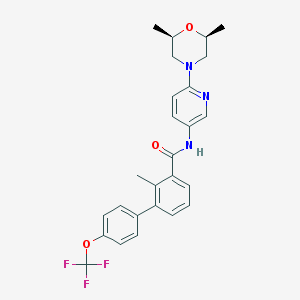

SMILE Code:
C[C@@H]1CN(C[C@@H](O1)C)C2=NC=C(C=C2)NC(=O)C3=CC=CC(=C3C)C4=CC=C(C=C4)OC(F)(F)F
C[C@@H]1CN(C[C@@H](O1)C)C2=NC=C(C=C2)NC(=O)C3=CC=CC(=C3C)C4=CC=C(C=C4)OC(F)(F)F
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.