Search for drugs:
Typing the drug name to query
LISDEXAMFETAMINE DIMESYLATE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:2
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post approval use of VYVANSE. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events are as follows: cardiomyopathy, mydriasis, diplopia, difficulties with visual accommodation, blurred vision, eosinophilic hepatitis, anaphylactic reaction, hypersensitivity, dyskinesia, dysgeusia, tics, bruxism, depression, dermatillomania, alopecia, aggression, Stevens-Johnson Syndrome, chest pain, angioedema, urticaria, seizures, libido changes, frequent or prolonged erections, constipation, and rhabdomyolysis.
- OVERDOSAGE
- Consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice for treatment of overdosage. Individual patient response to amphetamines varies widely. Toxic symptoms may occur idiosyncratically at low doses.
- Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has been reported with amphetamine use, including VYVANSE. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
- Lisdexamfetamine and d-amphetamine are not dialyzable.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
18
42894
Other ADRs
8628
14108651
Odds Ratio = 0.687
Drug Property Information
ATC Code(s):
- N06BA12 - lisdexamfetamine dimesylate
- N06BA - Centrally acting sympathomimetics
- N06B - "PSYCHOSTIMULANTS, AGENTS USED FOR ADHD AND NOOTROPICS"
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:lisdexamfetamine dimesylate
Active Ingredient UNII:SJT761GEGS
Drugbank ID:DB01255
PubChem Compound:11597698
CAS Number:608137-32-2
Dosage Form(s):capsule; tablet, chewable
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day N06BA12
Chemical Structure: 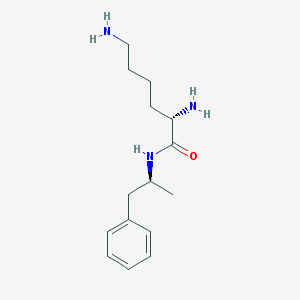

SMILE Code:
C[C@@H](CC1=CC=CC=C1)NC(=O)[C@H](CCCCN)N
C[C@@H](CC1=CC=CC=C1)NC(=O)[C@H](CCCCN)N
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.