Search for drugs:
Typing the drug name to query
DONEPEZIL HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Postmarketing Experience
- The following adverse reactions have been identified during post-approval use donepezil hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Abdominal pain, agitation, aggression, cholecystitis, confusion, convulsions, hallucinations, heart block (all types), hemolytic anemia, hepatitis, hyponatremia, neuroleptic malignant syndrome, pancreatitis, rash, rhabdomyolysis, QTc prolongation, and torsade de pointes.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
182
42730
Other ADRs
8137
14109142
Odds Ratio = 7.386
Drug Property Information
ATC Code(s):
- N06DA02 - donepezil hydrochloride
- N06DA - Anticholinesterases
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- N06DA52 - donepezil hydrochloride
- N06DA - Anticholinesterases
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
- N06DA53 - donepezil hydrochloride
- N06DA - Anticholinesterases
- N06D - ANTI-DEMENTIA DRUGS
- N06 - PSYCHOANALEPTICS
- N - NERVOUS SYSTEM
Active Ingredient:donepezil hydrochloride
Active Ingredient UNII:3O2T2PJ89D
Drugbank ID:DB00843
PubChem Compound:3152
CAS Number:120014-06-4
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 7.5 mg/day N06DA02
Chemical Structure: 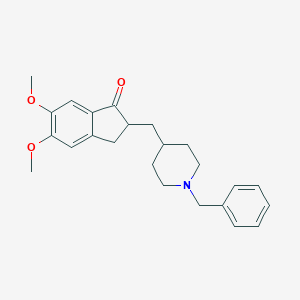

SMILE Code:
COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC
COC1=C(C=C2C(=C1)CC(C2=O)CC3CCN(CC3)CC4=CC=CC=C4)OC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.