Search for drugs:
Typing the drug name to query
VALSARTAN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-Marketing Experience
- Rare cases of rhabdomyolysis have been reported in patients receiving angiotensin II receptor blockers.
- Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
138
42774
Other ADRs
21438
14095841
Odds Ratio = 2.122
Drug Property Information
ATC Code(s):
- C09DX02 - valsartan
- C09DX -
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DB08 - valsartan
- C09DB - Angiotensin II antagonists and calcium channel blockers
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C10BX10 - valsartan
- C10BX - "HMG CoA reductase inhibitors, other combinations"
- C10B - "LIPID MODIFYING AGENTS, COMBINATIONS"
- C10 - LIPID MODIFYING AGENTS
- C - CARDIOVASCULAR SYSTEM
- C09DB01 - valsartan
- C09DB - Angiotensin II antagonists and calcium channel blockers
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DX01 - valsartan
- C09DX -
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DA03 - valsartan
- C09DA - Angiotensin II antagonists and diuretics
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09DX04 - valsartan
- C09DX -
- C09D - "ANGIOTENSIN II ANTAGONISTS, COMBINATIONS"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
- C09CA03 - valsartan
- C09CA - "Angiotensin II antagonists, plain"
- C09C - "ANGIOTENSIN II ANTAGONISTS, PLAIN"
- C09 - AGENTS ACTING ON THE RENIN-ANGIOTENSIN SYSTEM
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:valsartan
Active Ingredient UNII:80M03YXJ7I
Drugbank ID:DB00177
PubChem Compound:60846
CAS Number:137862-53-4
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 80.0 mg/day C09CA03
Chemical Structure: 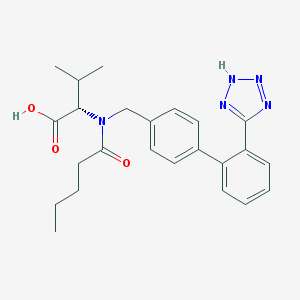

SMILE Code:
CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)[C@@H](C(C)C)C(=O)O
CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NNN=N3)[C@@H](C(C)C)C(=O)O
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: Rhabdomyolysis After Coadministration of Atorvastatin and Sacubitril/Valsartan (Entresto™) in a 63-Year-Old Woman.
[Faber Eve S,Gavini Madhavi,Ramirez Ronald,Sadovsky Richard]Drug Saf Case Rep.2016 Dec;3(1):14. PMID: 27804100
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.