Search for drugs:
Typing the drug name to query
COLCHICINE
DIR Classification
Classification:Moderate-DIR concern
Severity Score:3
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Neuromuscular Toxicity
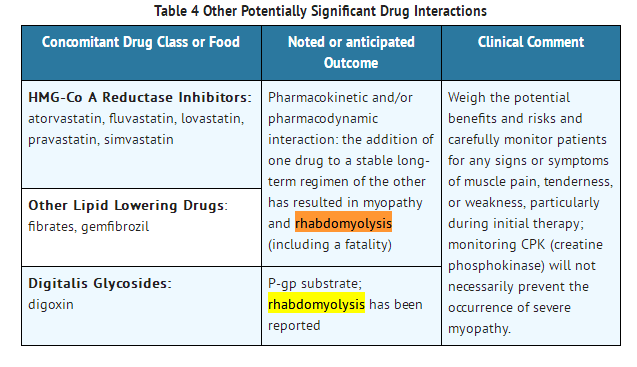
- Colchicine-induced neuromuscular toxicity and rhabdomyolysis have been reported with chronic treatment in therapeutic doses. Patients with renal dysfunction and elderly patients, even those with normal renal and hepatic function, are at increased risk. Concomitant use of atorvastatin, simvastatin, pravastatin, fluvastatin, lovastatin, gemfibrozil, fenofibrate, fenofibric acid, or benzafibrate (themselves associated with myotoxicity) or cyclosporine with COLCRYS may potentiate the development of myopathy [see DRUG INTERACTIONS (7)]. Once colchicine is stopped, the symptoms generally resolve within 1 week to several months.
- ADVERSE REACTIONS
- Postmarketing Experience
- Serious toxic manifestations associated with colchicine include myelosuppression, disseminated intravascular coagulation, and injury to cells in the renal, hepatic, circulatory, and central nervous systems.
- These most often occur with excessive accumulation or overdosage [see OVERDOSAGE (10)].
- The following adverse reactions have been reported with colchicine. These have been generally reversible upon temporarily interrupting treatment or lowering the dose of colchicine.
- Neurological: sensory motor neuropathy
- Dermatological: alopecia, maculopapular rash, purpura, rash
- Digestive: abdominal cramping, abdominal pain, diarrhea, lactose intolerance, nausea, vomiting
- Hematological: leukopenia, granulocytopenia, thrombocytopenia, pancytopenia, aplastic anemia
- Hepatobiliary: elevated AST, elevated ALT
- Musculoskeletal: myopathy, elevated CPK, myotonia, muscle weakness, muscle pain, rhabdomyolysis
- Reproductive: azoospermia, oligospermia
- DRUG INTERACTIONS
- COLCRYS (colchicine) is a substrate of the efflux transporter P-glycoprotein (P-gp). Of the cytochrome P450 enzymes tested, CYP3A4 was mainly involved in the metabolism of colchicine. If COLCRYS is administered with drugs that inhibit P-gp, most of which also inhibit CYP3A4, increased concentrations of colchicine are likely. Fatal drug interactions have been reported.
- Physicians should ensure that patients are suitable candidates for treatment with COLCRYS and remain alert for signs and symptoms of toxicities related to increased colchicine exposure as a result of a drug interaction. Signs and symptoms of COLCRYS toxicity should be evaluated promptly and, if toxicity is suspected, COLCRYS should be discontinued immediately.
- Table 4 provides recommendations as a result of other potentially significant drug interactions. Table 1 provides recommendations for strong and moderate CYP3A4 inhibitors and P-gp inhibitors.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
62
42850
Other ADRs
1456
14115823
Odds Ratio = 14.028
Drug Property Information
ATC Code(s):
- M04AC01 - colchicine
- M04AC - Preparations with no effect on uric acid metabolism
- M04A - ANTIGOUT PREPARATIONS
- M04 - ANTIGOUT PREPARATIONS
- M - MUSCULO-SKELETAL SYSTEM
Active Ingredient:colchicine
Active Ingredient UNII:SML2Y3J35T
Drugbank ID:DB01394
PubChem Compound:2833
CAS Number:64-86-8
Dosage Form(s):tablet, film coated
Route(s) Of Administrator:oral
Daily Dose:
- 1.0 mg/day M04AC01
Chemical Structure: 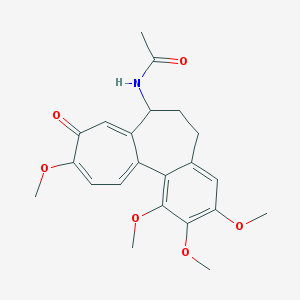

SMILE Code:
CC(=O)NC1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC
CC(=O)NC1CCC2=CC(=C(C(=C2C3=CC=C(C(=O)C=C13)OC)OC)OC)OC
Reference
COHORT STUDY:
1: Rhabdomyolysis: an evaluation of 475 hospitalized patients.
[Melli G, Chaudhry V, Cornblath DR, Medicine (Baltimore). 2005 Nov;84(6):377-85.]ABSTRACT
Rhabdomyolysis is a common and potentially lethal clinical syndrome that results from acute muscle fiber necrosis with leakage of muscle constituents into blood. Myoglobinuria is the most significant consequence, leading to acute renal failure (ARF) in 15%-33% of patients with rhabdomyolysis. Rhabdomyolysis occurs from inherited diseases, toxins, muscle compression or overexertion, or inflammatory processes, among other disorders. In some cases, no cause is found. We describe 475 patients from the Johns Hopkins Hospital inpatient records between January 1993 and December 2001 for the following discharge diagnosis codes: myoglobinuria, rhabdomyolysis, myopathy, toxic myopathy, malignant hyperthermia, neuroleptic malignant syndrome, and polymyositis. Of 1362 patients, 475 patients with an acute neuromuscular illness with serum creatine kinase (CK) more than 5 times the upper limit of normal (>975 IU/L) were included. Patients with recent myocardial infarction or stroke were excluded. The etiology was assigned by chart review. For all, the highest values of serum CK, serum creatinine and urine myoglobin, hemoglobin, and red blood cells were recorded. Forty-one patients had muscle biopsy within at least 2 months from the onset of rhabdomyolysis.Of the 475 patients, 151 were female and 324 were male (median age, 47 yr; range, 4-95 yr). Exogenous toxins were the most common cause of rhabdomyolysis, with illicit drugs, alcohol, and prescribed drugs responsible for 46%. Among the medical drugs, antipsychotics, statins, zidovudine, colchicine, selective serotonin reuptake inhibitors, and lithium were the most frequently involved. In 60% of all cases, multiple factors were present. In 11% of all cases, rhabdomyolysis was recurrent. Underlying myopathy or muscle metabolic defects were responsible for 10% of cases, in which there was a high percentage of recurrence, only 1 etiologic factor, and a low incidence of ARF. In 7%, no cause was found. ARF was present in 218 (46%) patients, and 16 died (3.4%). A linear correlation was found between CK and creatinine and between multiple factors and ARF, but there was no correlation between ARF and death or between multiple factors and death. Urine myoglobin detected by dipstick/ultrafiltration was positive in only 19%. Toxins are the most frequent cause of rhabdomyolysis, but in most cases more than 1 etiologic factor was present. Patients using illicit drugs or on prescribed polytherapy are at risk for rhabdomyolysis. The absence of urine myoglobin, by qualitative assay, does not exclude rhabdomyolysis. With appropriate care, death is rare.
PMID: 16267412
OTHER REFERENCE(S):
1: Severe hypertriglyceridemia and colchicine intoxication following suicide attempt.
[Lev Shaul,Snyder David,Azran Carmil,Zolotarsky Victor,Dahan Arik]Drug Des Devel Ther.2017 Nov 22;11:3321-3324. doi: 10.2147/DDDT.S140574. eCollection 2017. PMID: 29200827
2: Colchicine intoxication in familial Mediterranean fever patients using clarithromycin for the treatment of Helicobacter pylori: a series of six patients.
[Haj Yahia Soad,Ben Zvi Ilan,Livneh Avi]Rheumatol Int.2018 Jan;38(1):141-147. doi: 10.1007/s00296-017-3823-1. Epub 2017 Oct 3. PMID: 28975396
3: Colchicine triggered severe rhabdomyolysis after long-term low-dose simvastatin therapy: a case report.
[Frydrychowicz Clara,Pasieka Bastian,Pierer Matthias,Mueller Wolf,Petros Sirak,Weidhase Lorenz]J Med Case Rep.2017 Jan 4;11(1):8. doi: 10.1186/s13256-016-1169-z. PMID: 28049514
4: Poster 97 Statin-Induced Rhabdomyolysis Triggered by Concomitant Colchicine Administration: A Case Report.
[Auriemma Michael J,Bunning Robert D]PM R.2016 Sep;8(9S):S193. doi: 10.1016/j.pmrj.2016.07.140. Epub 2016 Sep 24. PMID: 27672864
5: An Unexpected Interaction between Sofosbuvir/Ledipasvir and Atorvastatin and Colchicine Causing Rhabdomyolysis in a Patient with Impaired Renal Function.
[Patel Shyam,Andres Jennifer,Qureshi Kamran]Case Rep Med.2016;2016:3191089. doi: 10.1155/2016/3191089. Epub 2016 Aug 22. PMID: 27635145
6: Colchicine-Induced Rhabdomyolysis: An Autopsy Case.
[Arslan Murat Nihat,Özgün Ayşe,Daş Taner,Kumru Durmuş,Şam Bülent,Koç Sermet]Am J Forensic Med Pathol.2016 Jun;37(2):57-9. doi: 10.1097/PAF.0000000000000225. PMID: 27049658
7: Thiocolchicoside: review of adverse effects.
Prescrire Int.2016 Feb;25(168):41-3. PMID: 27042729
8: Progressive Organ Failure After Ingestion of Wild Garlic Juice.
[Galland-Decker Coralie,Charmoy Alexia,Jolliet Philippe,Spertini Olivier,Hugli Olivier,Pantet Olivier]J Emerg Med.2016 Jan;50(1):55-60. doi: 10.1016/j.jemermed.2015.06.034. Epub 2015 Aug 15. PMID: 26281812
9: Colchicine-clarithromycin-induced rhabdomyolysis in Familial Mediterranean Fever patients under treatment for Helicobacter pylori.
[Cohen Oren,Locketz Garrett,Hershko Alon Y,Gorshtein Alexander,Levy Yair]Rheumatol Int.2015 Nov;35(11):1937-41. doi: 10.1007/s00296-015-3325-y. Epub 2015 Jul 26. PMID: 26210999
10: Clarithromycin-associated rhabdomyolysis in an infant.
[Mustafa Gulgun,Necati Balamtekin]J Clin Rheumatol.2014 Dec;20(8):457. doi: 10.1097/RHU.0000000000000192. PMID: 25417692
11: Acute colchicine intoxication complicated with extramedullary hematopoiesis due to filgrastim in a child.
[Kilic Suar C,Alaygut Demet,Unal Ekrem,Koç Elif,Patiroglu Turkan]J Pediatr Hematol Oncol.2014 Oct;36(7):e460-2. doi: 10.1097/MPH.0000000000000071. PMID: 24309614
12: Colchicine-induced rhabdomyolysis following a concomitant use of clarithromycin in a haemodialysis patient with familial Mediterranean fever.
[Çelebi Zeynep Kendi,Akturk Serkan,Oktay Esen Ismet,Duman Neval,Keven Kenan]Clin Kidney J.2013 Dec;6(6):665-6. doi: 10.1093/ckj/sft129. PMID: 26120465
13: Characterization of Statin-Associated Myopathy Case Reports in Thailand Using the Health Product Vigilance Center Database.
[Boonmuang Pornwalai,Nathisuwan Surakit,Chaiyakunapruk Nathorn,Suwankesawong Wimon,Pokhagul Pattreya,Teerawattanapong Nattawat,Supsongserm Pairin]Drug Saf.2013 Sep;36(9):779-87. doi: 10.1007/s40264-013-0055-5. PMID: 23615756
14: Cytochrome P450 drug interactions with statin therapy.
[Goh Ivanna Xin Wei,How Choon How,Tavintharan Subramaniam]Singapore Med J.2013 Mar;54(3):131-5. PMID: 23546024
15: Colchicine-induced rhabdomyolysis caused by interaction with clarithromycin in a patient with Behcet disease.
[Kim Ji-Beom,Kim Sujeong,Lee Taehoon,Lee Yoon Su,Cho You Sook,Moon Hee-Bom,Kim Yong-Gil,Kim Tae-Bum]J Clin Rheumatol.2013 Mar;19(2):108-9. doi: 10.1097/RHU.0b013e31828639e0. PMID: 23425672
16: Colchicine-induced rhabdomyolysis caused by interaction with clarithromycin in a patient with Behçet disease.
[Kim Ji-Beom,Kim Sujeong,Yoon Sun-young,Lee Taehoon,Lee Yoon Su,Kwon Hyouk-Soo,Cho You Sook,Moon Hee-Bom,Kim Yong-Gil,Kim Tae-Bum]J Clin Rheumatol.2012 Dec;18(8):453-4. doi: 10.1097/RHU.0b013e318279304e. PMID: 23211591
17: Severe colchicine intoxication in a renal transplant recipient on cyclosporine.
[Garrouste C,Philipponnet C,Kaysi S,Enache I,Tiple A,Heng A E]Transplant Proc.2012 Nov;44(9):2851-2. doi: 10.1016/j.transproceed.2012.09.028. PMID: 23146540
18: Colchicine toxicity in end-stage renal disease patients: a case-control study.
[Solak Yalcin,Atalay Huseyin,Biyik Zeynep,Alibasic Hayrudin,Gaipov Abduzhappar,Guney Figen,Kucuk Adem,Tonbul Halil Zeki,Yeksan Mehdi,Turk Suleyman]Am J Ther.2014 Nov-Dec;21(6):e189-95. doi: 10.1097/MJT.0b013e31825a364a. PMID: 22874645
19: Colchicine-induced rhabdomyolysis in a heart/lung transplant patient with concurrent use of cyclosporin, pravastatin, and azithromycin.
[Bouquié Régis,Deslandes Guillaume,Renaud Christian,Dailly Eric,Haloun Alain,Jolliet Pascale]J Clin Rheumatol.2011 Jan;17(1):28-30. doi: 10.1097/RHU.0b013e3182056042. PMID: 21169852
20: Rhabdomyolysis induced by co-administration of fluvastatin and colchicine.
[Sarullo Filippo M,Americo Luigi,Di Franco Antonino,Di Pasquale Pietro]Monaldi Arch Chest Dis.2010 Sep;74(3):147-9. PMID: 21110512
21: Multiple organ failure after an overdose of less than 0.4 mg/kg of colchicine: role of coingestants and drugs during intensive care management.
[Montiel Virginie,Huberlant Vincent,Vincent Marie-Françoise,Bonbled Frédéric,Hantson Philippe]Clin Toxicol (Phila).2010 Oct;48(8):845-8. doi: 10.3109/15563650.2010.509101. Epub 2010 Aug 12. PMID: 20969505
22: Short term treatment with clarithromycin resulting in colchicine-induced rhabdomyolysis.
[McKinnell James,Tayek John A]J Clin Rheumatol.2009 Sep;15(6):303-5. doi: 10.1097/RHU.0b013e3181bbbcd7. PMID: 19734738
23: Colchicine-induced toxicity in a heart transplant patient with chronic renal failure.
[Eleftheriou Giorgio,Bacis Guiseppe,Fiocchi Roberto,Sebastiano Roberta]Clin Toxicol (Phila).2008 Nov;46(9):827-30. doi: 10.1080/15563650701779703. PMID: 18608282
24: Severe obstructive sleep apnea after cerivastatin therapy: a case report.
[Ebben Matthew R,Sethi Nitin K,Spielman Arthur J]J Clin Sleep Med.2008 Jun 15;4(3):255-6. PMID: 18595439
25: Rapid onset of muscle weakness (rhabdomyolysis) associated with the combined use of simvastatin and colchicine.
[Justiniano Maria,Dold Sylvia,Espinoza Luis R]J Clin Rheumatol.2007 Oct;13(5):266-8. PMID: 17921794
26: Which statin should be used together with colchicine? Clinical experience in three patients with nephrotic syndrome due to AA type amyloidosis.
[Sahin Garip,Korkmaz Cengiz,Yalcin Ahmet Uğur]Rheumatol Int.2008 Jan;28(3):289-91. Epub 2007 Aug 17. PMID: 17703308
27: Fatal toxic myopathy attributed to propofol, methylprednisolone, and cyclosporine after prior exposure to colchicine and simvastatin.
[Francis Lisa,Bonilla Eduardo,Soforo Ekaterina,Neupane Hom,Nakhla Hassan,Fuller Christine,Perl Andras]Clin Rheumatol.2008 Jan;27(1):129-31. Epub 2007 Jul 13. PMID: 17628739
28: Colchicine-induced rhabdomyolysis.
[Altman Arie,Szyper-Kravitz Martine,Shoenfeld Yehuda]Clin Rheumatol.2007 Dec;26(12):2197-2199. doi: 10.1007/s10067-007-0682-2. Epub 2007 Jul 10. PMID: 17619811
29: A complex case of renal amyloidosis with a rare co-occurrence of 2 mutations in separate hereditary periodic fever syndrome-related genes.
[Cigni Alessandro,Ledda Franca,Satta Andrea E]J Nephrol.2006 Jul-Aug;19(4):543-9. PMID: 17048217
30: Colchicine myoneuropathy: the role of rhabdomyolysis.
[Varughese George I,Varghese Abraham I]Nephrology (Carlton).2006 Oct;11(5):481-2; author reply 482. PMID: 17014570
31: Rhabdomyolysis in a patient treated with colchicine and atorvastatin.
[Tufan Abdurrahman,Dede Didem Sener,Cavus Safak,Altintas Neriman Defne,Iskit Alper Bektas,Topeli Arzu]Ann Pharmacother.2006 Jul-Aug;40(7-8):1466-9. Epub 2006 Jun 13. PMID: 16772404
32: Rhabdomyolysis: an evaluation of 475 hospitalized patients.
[Melli Giorgia,Chaudhry Vinay,Cornblath David R]Medicine (Baltimore).2005 Nov;84(6):377-85. PMID: 16267412
33: [A case report of acute neuromyopathy induced by concomitant use of colchicine and bezafibrate].
[Sugie Masayuki,Kuriki Ayako,Arai Daisuke,Ichikawa Hiroo,Kawamura Mitsuru]No To Shinkei.2005 Sep;57(9):785-90. PMID: 16248366
34: Acute renal failure associated with an accidental overdose of colchicine.
[Borrás-Blasco J,Enriquez R,Sirvent A E,Amoros F,Navarro-Ruiz A,Reyes A]Int J Clin Pharmacol Ther.2005 Oct;43(10):480-4. PMID: 16240705
35: Possible colchicine rhabdomyolysis in a fluvastatin-treated patient.
[Atasoyu Enes Murat,Evrenkaya T Rifki,Solmazgul Emrullah]Ann Pharmacother.2005 Jul-Aug;39(7-8):1368-9. Epub 2005 Jun 14. PMID: 15956236
36: Cytoskeletal myotoxicity from simvastatin and colchicine.
[Baker Steven K,Goodwin Susan,Sur Monalisa,Tarnopolsky Mark A]Muscle Nerve.2004 Dec;30(6):799-802. PMID: 15389652
37: Case report: fatal poisoning with Colchicum autumnale.
[Brvar Miran,Ploj Tom,Kozelj Gordana,Mozina Martin,Noc Marko,Bunc Matjaz]Crit Care.2004 Feb;8(1):R56-9. Epub 2004 Jan 2. PMID: 14975056
38: Colchicine-induced rhabdomyolysis in a patient with chronic heart failure.
[Debie Karen,Conraads Viviane,Vrints Christiaan]Acta Cardiol.2003 Dec;58(6):561-2. PMID: 14713183
39: Colchicine-induced myopathy with myotonia in a patient with chronic renal failure.
[Caglar Kayser,Odabasi Zeki,Safali Mukerrem,Yenicesu Mujdat,Vural Abdulgaffar]Clin Neurol Neurosurg.2003 Sep;105(4):274-6. PMID: 12954545
40: Colchicine-induced rhabdomyolysis: the whole is greater than the sum of its parts!
[Vasudevan Abu R,Uthamalingam Shanmugham,Kumar Sajal,Tamarin Frank,Brensilver Jeffrey M]Am J Med.2003 Aug 15;115(3):249. PMID: 12935834
41: Colchicine-induced rhabdomyolysis.
[Phanish Mysore K,Krishnamurthy Suchitra,Bloodworth Lionel L O]Am J Med.2003 Feb 1;114(2):166-7. PMID: 12586247
42: Rhabdomyolysis associated with gemfibrozil-colchicine therapy.
[Atmaca Hulusi,Sayarlioglu Hayriye,Külah Eyüp,Demircan Nejat,Akpolat Tekin]Ann Pharmacother.2002 Nov;36(11):1719-21. PMID: 12398566
43: Colchicine-induced rhabdomyolysis.
[Boomershine Kellie H]Ann Pharmacother.2002 May;36(5):824-6. PMID: 11978160
44: Colchicine induced rhabdomyolysis.
[Chattopadhyay I,Shetty H G,Routledge P A,Jeffery J]Postgrad Med J.2001 Mar;77(905):191-2. PMID: 11222829
45: [Multi-systemic toxicity of colchicine and renal failure: apropos of a case].
[Rosset L,Descombes E,Fellay G,Regamey C]Schweiz Med Wochenschr.1998 Dec 5;128(49):1953-7. PMID: 9887475
46: Fatal colchicine overdose: report of a case and review of the literature.
[Milne S T,Meek P D]Am J Emerg Med.1998 Oct;16(6):603-8. PMID: 9786547
47: Colchicine induced rhabdomyolysis.
[Dawson T M,Starkebaum G]J Rheumatol.1997 Oct;24(10):2045-6. PMID: 9330953
48: Colchicine toxicity in patients with chronic renal failure.
[Montseny J J,Meyrier A,Gherardi R K]Nephrol Dial Transplant.1996 Oct;11(10):2055-8. PMID: 8918722
49: [Diarrhea and cytolysis].
[Verdier-Plas M,Buisson M,Lagnoui R,Bannwarth B,Ragnaud J M,Gin H,Aubertin J]Rev Med Interne.1995;16 Suppl 2:254s-256s. PMID: 7652248
50: [Toxic myopathy with kidney failure as a colchicine side effect ifn familial Mediterranean fever].
[Stefanidis I,Böhm R,Hägel J,Maurin N]Dtsch Med Wochenschr.1992 Aug 14;117(33):1237-40. PMID: 1499522
51: [Drug-induced rhabdomyolysis].
[Chichmanian R M,Mignot G,Spreux A]Ann Med Interne (Paris).1991;142(8):587-91. PMID: 1807179
52: Is myopathy in renal transplant patients induced by cyclosporin or colchicine?
[Rumpf K W,Henning H V]Lancet.1990 Mar 31;335(8692):800-1. PMID: 1969550
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.