Search for drugs:
Typing the drug name to query
ERLOTINIB HYDROCHLORIDE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- ADVERSE REACTIONS
- Post-Marketing Experience
- The following adverse reactions have been identified during post approval use of TARCEVA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Musculoskeletal and Connective Tissue Disorders: myopathy, including rhabdomyolysis, in combination with statin therapy
- Eye Disorders: ocular inflammation including uveitis
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
36
42876
Other ADRs
38808
14078471
Odds Ratio = 0.305
Drug Property Information
ATC Code(s):
- L01XE03 - erlotinib hydrochloride
- L01XE - Protein kinase inhibitors
- L01X - OTHER ANTINEOPLASTIC AGENTS
- L01 - ANTINEOPLASTIC AGENTS
- L - ANTINEOPLASTIC AND IMMUNOMODULATING AGENTS
Active Ingredient:erlotinib hydrochloride
Active Ingredient UNII:DA87705X9K
Drugbank ID:DB00530
PubChem Compound:176870
CAS Number:183321-74-6
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
Chemical Structure: 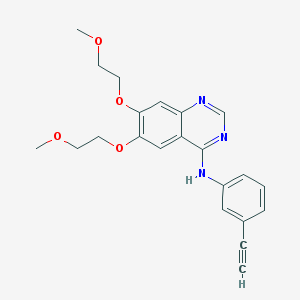

SMILE Code:
COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC
COCCOC1=C(C=C2C(=C1)C(=NC=N2)NC3=CC=CC(=C3)C#C)OCCOC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: A phase I study of high-dose rosuvastatin with standard dose erlotinib in patients with advanced solid malignancies.
[Goss Glenwood D,Jonker Derek J,Laurie Scott A,Weberpals Johanne I,Oza Amit M,Spaans Johanna N,la Porte Charles,Dimitroulakos Jim]J Transl Med.2016 Mar 31;14:83. doi: 10.1186/s12967-016-0836-6. PMID: 27036206
2: Erlotinib-related rhabdomyolysis: the role of pharmacogenetics and drug-drug interaction.
[Koršić Marta,Muršić Davorka,Badovinac Sonja,Božina Nada,Roglić Mihovil,Jakopović Marko,Čučević Branka]Cancer Chemother Pharmacol.2015 Dec;76(6):1317-9. doi: 10.1007/s00280-015-2885-6. Epub 2015 Oct 20. PMID: 26482820
3: Rhabdomyolysis from erlotinib: a case report.
[Moscetti Luca,Nelli Fabrizio,Ruggeri Enzo Maria]Tumori.2011 May-Jun;97(3):415-6. doi: 10.1700/912.10044. PMID: 21789026
4: Rhabdomyolysis resulting from pharmacologic interaction between erlotinib and simvastatin.
[Veeraputhiran Muthu,Sundermeyer Mark]Clin Lung Cancer.2008 Jul;9(4):232-4. doi: 10.3816/CLC.2008.n.036. PMID: 18650173
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.