Search for drugs:
Typing the drug name to query
TOLVAPTAN
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- In a parallel-arm, double-blind (for tolvaptan and placebo), placebo- and positive-controlled, multiple dose study of the effect of tolvaptan on the QTc interval, 172 healthy subjects were randomized to tolvaptan 30 mg, tolvaptan 300 mg, placebo, or moxifloxacin 400 mg once daily. At both the 30 mg and 300 mg doses, no significant effect of administering tolvaptan on the QTc interval was detected on Day 1 and Day 5. At the 300 mg dose, peak tolvaptan plasma concentrations were approximately 4-fold higher than the peak concentrations following a 30 mg dose. Moxifloxacin increased the QT interval by 12 ms at 2 hours after dosing on Day 1 and 17 ms at 1 hour after dosing on Day 5, indicating that the study was adequately designed and conducted to detect tolvaptan’s effect on the QT interval, had an effect been present.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
3
42909
Other ADRs
3384
14113895
Odds Ratio = 0.292
Drug Property Information
ATC Code(s):
- C03XA01 - tolvaptan
- C03XA - Vasopressin antagonists
- C03X - OTHER DIURETICS
- C03 - DIURETICS
- C - CARDIOVASCULAR SYSTEM
Active Ingredient:tolvaptan
Active Ingredient UNII:21G72T1950
Drugbank ID:DB06212
PubChem Compound:443894
CAS Number:150683-30-0
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 30.0 mg/day C03XA01
Chemical Structure: 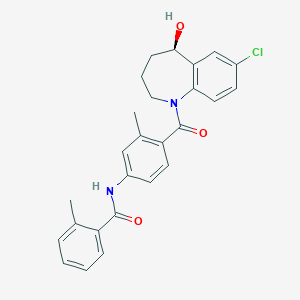

SMILE Code:
CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCC[C@H](C4=C3C=CC(=C4)Cl)O)C
CC1=CC=CC=C1C(=O)NC2=CC(=C(C=C2)C(=O)N3CCC[C@H](C4=C3C=CC(=C4)Cl)O)C
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: [Severe hyponatremia secondary to the syndrome of inappropriate secretion of antidiuretic hormone].
[Foppiani Luca,Raggi Francesca,Tagliabue Milena,Benso Andrea,Brandolin Irene,Lo Pinto Giuliano]Recenti Prog Med.2013 Mar;104(3):112-5. doi: 10.1701/1255.13859. PMID: 23548955
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.