Search for drugs:
Typing the drug name to query
FEBUXOSTAT
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CLINICAL PHARMACOLOGY
- Pharmacodynamics
- Effect on Cardiac Repolarization:
- The effect of febuxostat on cardiac repolarization as assessed by the QTc interval was evaluated in normal healthy patients and in patients with gout. Febuxostat in doses up to 300 mg daily (3.75 times the maximum recommended daily dosage), at steady-state, did not demonstrate an effect on the QTc interval.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
33
42879
Other ADRs
3497
14113782
Odds Ratio = 3.107
Drug Property Information
ATC Code(s):
- M04AA03 - febuxostat
- M04AA - Preparations inhibiting uric acid production
- M04A - ANTIGOUT PREPARATIONS
- M04 - ANTIGOUT PREPARATIONS
- M - MUSCULO-SKELETAL SYSTEM
Active Ingredient:febuxostat
Active Ingredient UNII:101V0R1N2E
Drugbank ID:DB04854
PubChem Compound:134018
CAS Number:144060-53-7
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 80.0 mg/day M04AA03
Chemical Structure: 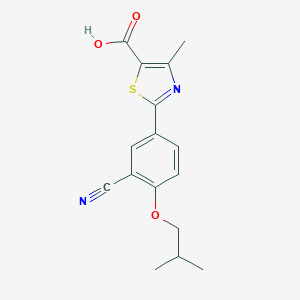

SMILE Code:
CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O
CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O
Reference
COHORT STUDY:
1: Risk of Febuxostat-Associated Myopathy in Patients with CKD.
[Liu CT, Chen CY, Hsu CY, Huang PH, Lin FY, Chen JW, Lin SJ, Clin J Am Soc Nephrol. 2017 May 8;12(5):744-750.]ABSTRACT
BACKGROUND AND OBJECTIVES: Febuxostat, a nonpurine xanthine oxidase inhibitor, is widely used to treat hyperuricemia. Although febuxostat-associated rhabdomyolysis was reported in some patients with CKD, the association between CKD and febuxostat-associated myopathy remains uncertain.
DESIGN, SETTING, PARTICIPANTS, & MEASUREMENTS: Our retrospective cohort study included 1332 patients using febuxostat in Taipei Medical University-Wanfang Hospital from February of 2014 to January of 2016. The primary predictor was time-averaged eGFR as calculated by the equation proposed by the 2009 Chronic Kidney Disease Epidemiology Collaboration. The outcome was febuxostat-associated myopathy defined as elevated creatine kinase levels during febuxostat use that were not attributed to other muscular injuries.
RESULTS: The median duration of febuxostat use was 224 days (25th, 75th percentiles: 86, 441.5 days). Of 1332 study participants, 1222 (91.7%) had CKD; the median eGFR was 20.8 ml/min per 1.73 m2 (25th, 75th percentiles: 9.0, 35.4 ml/min per 1.73 m2). Forty-one of the participants had febuxostat-associated myopathy (3.2%). All patients with myopathy had CKD, and the incident rate was 0.013 (95% confidence interval, 0.01 to 0.02) events per 100 patient-days in patients with CKD. Of 41 patients with myopathy, 37 had myositis, and four had rhabdomyolysis. Myopathy resolved in 17 patients who withdrew from treatment and eight patients who continued febuxostat treatment. Among the evaluated predictors, multivariate analysis showed that only the lowest eGFR tertile was significantly associated with myopathy in febuxostat users. The odds ratio of the lowest eGFR tertile to the highest tertile was 4.21 (95% confidence interval, 1.7 to 10.43). This finding remained consistent among subgroups stratified by age, sex, diabetes status, coronary artery disease, and statin or fibrate use.
CONCLUSIONS: Patients with severely reduced eGFR had higher risk of myopathy with treatment of febuxostat. Regular monitoring of creatine kinase level is suggested for early detection of febuxostat-associated myopathy, particularly in patients with CKD.
PMID: 28302902
DESIGN, SETTING, PARTICIPANTS, & MEASUREMENTS: Our retrospective cohort study included 1332 patients using febuxostat in Taipei Medical University-Wanfang Hospital from February of 2014 to January of 2016. The primary predictor was time-averaged eGFR as calculated by the equation proposed by the 2009 Chronic Kidney Disease Epidemiology Collaboration. The outcome was febuxostat-associated myopathy defined as elevated creatine kinase levels during febuxostat use that were not attributed to other muscular injuries.
RESULTS: The median duration of febuxostat use was 224 days (25th, 75th percentiles: 86, 441.5 days). Of 1332 study participants, 1222 (91.7%) had CKD; the median eGFR was 20.8 ml/min per 1.73 m2 (25th, 75th percentiles: 9.0, 35.4 ml/min per 1.73 m2). Forty-one of the participants had febuxostat-associated myopathy (3.2%). All patients with myopathy had CKD, and the incident rate was 0.013 (95% confidence interval, 0.01 to 0.02) events per 100 patient-days in patients with CKD. Of 41 patients with myopathy, 37 had myositis, and four had rhabdomyolysis. Myopathy resolved in 17 patients who withdrew from treatment and eight patients who continued febuxostat treatment. Among the evaluated predictors, multivariate analysis showed that only the lowest eGFR tertile was significantly associated with myopathy in febuxostat users. The odds ratio of the lowest eGFR tertile to the highest tertile was 4.21 (95% confidence interval, 1.7 to 10.43). This finding remained consistent among subgroups stratified by age, sex, diabetes status, coronary artery disease, and statin or fibrate use.
CONCLUSIONS: Patients with severely reduced eGFR had higher risk of myopathy with treatment of febuxostat. Regular monitoring of creatine kinase level is suggested for early detection of febuxostat-associated myopathy, particularly in patients with CKD.
OTHER REFERENCE(S):
1: Risk of Febuxostat-Associated Myopathy in Patients with CKD.
[Liu Chung-Te,Chen Chun-You,Hsu Chien-Yi,Huang Po-Hsun,Lin Feng-Yen,Chen Jaw-Wen,Lin Shing-Jong]Clin J Am Soc Nephrol.2017 May 8;12(5):744-750. doi: 10.2215/CJN.08280816. Epub 2017 Mar 16. PMID: 28302902
2: Febuxostat-associated eosinophilic polymyositis in marginal zone lymphoma.
[Chahine Georges,Saleh Khalil,Ghorra Claude,Khoury Nathalie,Khalife Nadine,Fayad Fouad]Joint Bone Spine.2017 Mar;84(2):221-223. doi: 10.1016/j.jbspin.2016.10.008. Epub 2016 Dec 7. PMID: 27955822
3: Febuxostat-associated rhabdomyolysis in chronic renal failure.
[Ghosh Debasish,McGann Philippa M,Furlong Timothy J,Day Richard O]Med J Aust.2015 Jul 20;203(2):107-8. PMID: 26175252
4: Rhabdomyolysis associated with initiation of febuxostat therapy for hyperuricaemia in a patient with chronic kidney disease.
[Kang Y,Kim M J,Jang H N,Bae E J,Yun S,Cho H S,Chang S-H,Park D J]J Clin Pharm Ther.2014 Jun;39(3):328-30. doi: 10.1111/jcpt.12144. Epub 2014 Mar 10. PMID: 24612195
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.