Search for drugs:
Typing the drug name to query
RABEPRAZOLE SODIUM
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- CONTRAINDICATIONS
- Concomitant use of clarithromycin with pimozide and cisapride
- Concomitant administration of clarithromycin with pimozide and cisapride is contraindicated. There have been post-marketing reports of drug interactions when clarithromycin and/or erythromycin are co-administered with pimozide resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsade de pointes) most likely due to inhibition of hepatic metabolism of pimozide by erythromycin and clarithromycin. Fatalities have been reported. (Please refer to full prescribing information for clarithromycin.)
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
18
42894
Other ADRs
3970
14113309
Odds Ratio = 1.492
Drug Property Information
ATC Code(s):
- A02BC04 - rabeprazole sodium
- A02BC - Proton pump inhibitors
- A02B - DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
- A02 - DRUGS FOR ACID RELATED DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
- M01AA05 - rabeprazole sodium
- M01AA - Butylpyrazolidines
- M01A - "ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS, NON-STEROIDS"
- M01 - ANTIINFLAMMATORY AND ANTIRHEUMATIC PRODUCTS
- M - MUSCULO-SKELETAL SYSTEM
- M02AA03 - rabeprazole sodium
- M02AA - "Antiinflammatory preparations, non-steroids for topical use"
- M02A - TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
- M02 - TOPICAL PRODUCTS FOR JOINT AND MUSCULAR PAIN
- M - MUSCULO-SKELETAL SYSTEM
- A02BC54 - rabeprazole sodium
- A02BC - Proton pump inhibitors
- A02B - DRUGS FOR PEPTIC ULCER AND GASTRO-OESOPHAGEAL REFLUX DISEASE (GORD)
- A02 - DRUGS FOR ACID RELATED DISORDERS
- A - ALIMENTARY TRACT AND METABOLISM
Active Ingredient:rabeprazole sodium
Active Ingredient UNII:3L36P16U4R
Drugbank ID:DB01129
PubChem Compound:5029
CAS Number:117976-89-3
Dosage Form(s):tablet, delayed release
Route(s) Of Administrator:oral
Daily Dose:
- 20.0 mg/day A02BC04
Chemical Structure: 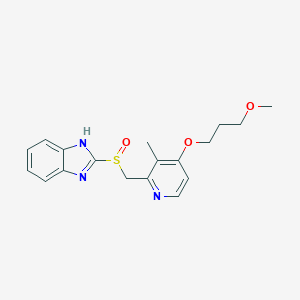

SMILE Code:
CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCCCOC
CC1=C(C=CN=C1CS(=O)C2=NC3=CC=CC=C3N2)OCCCOC
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
N/ADisclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.