Search for drugs:
Typing the drug name to query
NEVIRAPINE
DIR Classification
Classification:Less-DIR concern
Severity Score:1
Description in Drug Labeling: View Full Labeling: SPL in DailyMed | PDF
- WARNINGS AND PRECAUTIONS
- Hepatotoxicity and Hepatic Impairment
- Severe, life-threatening, and in some cases fatal hepatotoxicity, including fulminant and cholestatic hepatitis, hepatic necrosis and hepatic failure, have been reported in patients treated with Nevirapine. In controlled clinical trials, symptomatic hepatic events regardless of severity occurred in 4% (range 0% to 11%) of subjects who received Nevirapine and 1% of subjects in control groups.
- The risk of symptomatic hepatic events regardless of severity was greatest in the first 6 weeks of therapy. The risk continued to be greater in the Nevirapine groups compared to controls through 18 weeks of treatment. However, hepatic events may occur at any time during treatment. In some cases, subjects presented with non-specific, prodromal signs or symptoms of fatigue, malaise, anorexia, nausea, jaundice, liver tenderness or hepatomegaly, with or without initially abnormal serum transaminase levels. Rash was observed in approximately half of the subjects with symptomatic hepatic adverse events. Fever and flu-like symptoms accompanied some of these hepatic events. Some events, particularly those with rash and other symptoms, have progressed to hepatic failure with transaminase elevation, with or without hyperbilirubinemia, hepatic encephalopathy, prolonged partial thromboplastin time, or eosinophilia. Rhabdomyolysis has been observed in some patients experiencing skin and/or liver reactions associated with Nevirapine use. Patients with signs or symptoms of hepatitis must be advised to discontinue Nevirapine and immediately seek medical evaluation, which should include liver enzyme tests.
- Skin Reaction
- Severe and life-threatening skin reactions, including fatal cases, have been reported, occurring most frequently during the first 6 weeks of therapy. These have included cases of Stevens-Johnson syndrome, toxic epidermal necrolysis, and hypersensitivity reactions characterized by rash, constitutional findings, and organ dysfunction including hepatic failure. Rhabdomyolysis has been observed in some patients experiencing skin and/or liver reactions associated with Nevirapine use. In controlled clinical trials, Grade 3 and 4 rashes were reported during the first 6 weeks in 2% of Nevirapine recipients compared to less than 1% of placebo subjects.
- Patients developing signs or symptoms of severe skin reactions or hypersensitivity reactions (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial edema, and/or hepatitis, eosinophilia, granulocytopenia, lymphadenopathy, and renal dysfunction) must permanently discontinue Nevirapine and seek medical evaluation immediately [see BOXED WARNING and PATIENT COUNSELING INFORMATION (17.1)]. Do not restart Nevirapine following severe skin rash, skin rash combined with increased transaminases or other symptoms, or hypersensitivity reaction.
- If patients present with a suspected Nevirapine-associated rash, measure transaminases immediately. Permanently discontinue Nevirapine in patients with rash-associated transaminase elevations [see WARNINGS AND PRECAUTIONS (5.1)].
- Therapy with Nevirapine must be initiated with a 14-day lead-in period of 200 mg/day (150 mg/m2/day in pediatric patients), which has been shown to reduce the frequency of rash. Discontinue Nevirapine if a patient experiences severe rash or any rash accompanied by constitutional findings. Do not increase Nevirapine dose to a patient experiencing a mild to moderate rash without constitutional symptoms during the 14-day lead-in period of 200 mg/day (150 mg/m2/day in pediatric patients) until the rash has resolved. The total duration of the once-daily lead-in dosing period must not exceed 28 days at which point an alternative regimen should be sought [see DOSAGE AND ADMINISTRATION (2.4)]. Patients must be monitored closely if isolated rash of any severity occurs. Delay in stopping Nevirapine treatment after the onset of rash may result in a more serious reaction.
- Women appear to be at higher risk than men of developing rash with Nevirapine.
- In a clinical trial, concomitant prednisone use (40 mg/day for the first 14 days of Nevirapine administration) was associated with an increase in incidence and severity of rash during the first 6 weeks of Nevirapine therapy. Therefore, use of prednisone to prevent Nevirapine-associated rash is not recommended.
- ADVERSE REACTIONS
- Post-Marketing Experience
- In addition to the adverse events identified during clinical trials, the following adverse reactions have been identified during post-approval use of Nevirapine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- - Body as a Whole: fever, somnolence, drug withdrawal [see DRUG INTERACTIONS (7)], redistribution/accumulation of body fat [see WARNINGS AND PRECAUTIONS(5.6)]
- - Gastrointestinal: vomiting
- - Liver and Biliary: jaundice, fulminant and cholestatic hepatitis, hepatic necrosis, hepatic failure
- - Hematology: anemia, eosinophilia, neutropenia
- - Investigations: decreased serum phosphorus
- - Musculoskeletal: arthralgia, rhabdomyolysis associated with skin and/or liver reactions Neurologic: paraesthesia
- - Skin and Appendages: allergic reactions including anaphylaxis, angioedema, bullous eruptions, ulcerative stomatitis and urticaria have all been reported. In addition, hypersensitivity syndrome and hypersensitivity reactions with rash associated with constitutional findings such as fever, blistering, oral lesions, conjunctivitis, facial edema, muscle or joint aches, general malaise, fatigue or significant hepatic abnormalities [see WARNINGS AND PRECAUTIONS (5.1)] plus one or more of the following: hepatitis, eosinophilia, granulocytopenia, lymphadenopathy, and/or renal dysfunction have been reported.
- In post-marketing surveillance anemia has been more commonly observed in children although development of anemia due to concomitant medication use cannot be ruled out.
Postmarketing Surveillance
Contingency Table:
Current Drug
Other Drugs
Rhabdomyolysis
44
42868
Other ADRs
9096
14108183
Odds Ratio = 1.592
Drug Property Information
ATC Code(s):
- J05AR05 - nevirapine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AG01 - nevirapine
- J05AG - Non-nucleoside reverse transcriptase inhibitors
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
- J05AR07 - nevirapine
- J05AR - "Antivirals for treatment of HIV infections, combinations"
- J05A - DIRECT ACTING ANTIVIRALS
- J05 - ANTIVIRALS FOR SYSTEMIC USE
- J - ANTIINFECTIVES FOR SYSTEMIC USE
Active Ingredient:nevirapine
Active Ingredient UNII:99DK7FVK1H
Drugbank ID:DB00238
PubChem Compound:4463
CAS Number:129618-40-2
Dosage Form(s):tablet
Route(s) Of Administrator:oral
Daily Dose:
- 400.0 mg/day J05AG01
Chemical Structure: 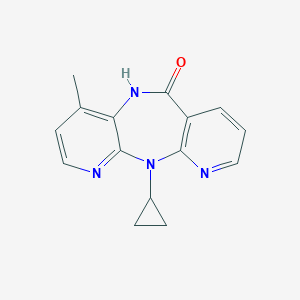

SMILE Code:
CC1=C2C(=NC=C1)N(C3=C(C=CC=N3)C(=O)N2)C4CC4
CC1=C2C(=NC=C1)N(C3=C(C=CC=N3)C(=O)N2)C4CC4
Reference
COHORT STUDY:
N/AOTHER REFERENCE(S):
1: No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database.
[Napoli Andrew A,Wood Jennifer J,Coumbis John J,Soitkar Amit M,Seekins Daniel W,Tilson Hugh H]J Int AIDS Soc.2014 Sep 4;17:19214. doi: 10.7448/IAS.17.1.19214. eCollection 2014. PMID: 25192857
2: From the Centers for Disease Control and Prevention. Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
JAMA.2001 Jan 24-31;285(4):402-3. PMID: 11263401
3: Serious adverse events attributed to nevirapine regimens for postexposure prophylaxis after HIV exposures--worldwide, 1997-2000.
[Centers for Disease Control and Prevention (CDC)]MMWR Morb Mortal Wkly Rep.2001 Jan 5;49(51-52):1153-6. PMID: 11198946
Disclaimer:
The content of this database of rhabdomyolysis is intended for educational and scientific research purposes only. It is not intended as a substitute for professional medical advice, diagnosis or treatment.
The views presented in this website do not necessarily reflect current or future opinion or policy of the US Food and Drug Administration. Any mention of commercial products is for clarification and not intended as endorsement.